Combination antiretroviral therapy (ART) revolutionized HIV care over the past 15 years converting a fatal disease to a treatable chronic condition. Despite this success, uncertainty remains regarding the optimal time to initiate ART. In 1998, the U.S. Department of Health and Human Services (DHHS) recommended an aggressive approach of starting ART at any CD4+ count <500 cells/mm3 (1). Concern over drug toxicity and accumulating resistance mutations motivated the DHHS panel to subsequently lower the CD4+ count threshold. With improved short term-safety of currently regimens, and appreciation that HIV infection may contribute to non-AIDS related conditions, the 2011 DHHS guidelines again recommend ART initiation at CD4+ counts ≤500 cells/mm3 and be considered at >500 cells/mm3—a threshold that would effectively mean offering treatment to everyone diagnosed with HIV infection—though only 50% of the panel supported the latter (2).
The study by the HIV-CAUSAL Collaboration in this issue (3) is the third recently published comprehensive cohort analysis to address the question of when to start ART (4, 5). This analysis reported increased mortality only when deferring ART initiation to a CD4+ count <200 cells/mm3. However, a benefit was seen when starting ART above 500 cells/mm3 by using a broader endpoint including non-fatal AIDS, with the notable caveat that these events are typically less severe at higher CD4+ counts (e.g., esophageal candidiasis). Previously, the When to Start Consortium reported increased risk for AIDS or death when ART initiation was deferred to <350 cells/mm3, while mortality risk was greater only when thresholds approached 200 cells/mm3 (4). In contrast, analyses by the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) reported a striking 94% increased risk of death when deferring ART to CD4+ counts <500 cells/mm3, even without including non-fatal AIDS events (5). Finally, recently presented data from the CASCADE collaboration also found a persistent, though diminishing, reduction in risk for AIDS or death with earlier ART initiation, but the number needed to treat to show benefit above the 500 cells/mm3 threshold was infinity (6). The differing results from these cohort studies may, in part, reflect differences in the statistical methods employed.
One important limitation from the current cohort data is the lack of outcomes related to serious non-fatal non-AIDS-defining conditions, which are the most common causes of morbidity and mortality at higher CD4+ counts (7). A widely held hypothesis is that persistent HIV-related inflammation may contribute to premature development of non-AIDS events (e.g., cardiovascular disease) and that earlier use of ART (with HIV suppression) will attenuate this risk. An important caveat is that a disproportionate amount of the HIV-related immune damage occurs very soon after initial infection (8) so that even early ART initiation (e.g. >500 cells/mm3) would be too late to reverse the process. Furthermore, it appears that ART may at best only partially attenuate inflammation while potentially adding ART-related toxicity (9, 10). Though risk for non-AIDS events has been inversely associated with CD4+ counts (11), the pathogenesis for these events is currently not well understood and likely differs by disease.
The other critical limitation inherent in all cohort studies is unmeasured confounding, which likely contributes to clinical decisions to start or defer ART in any given patient. This type of bias may have contributed to the large differences in mortality risk between the HIV-CAUSAL and NA-ACCORD results, and can only be addressed by a randomized trial. A randomized trial characterizing the benefits (both related to AIDS and non-AIDS events) of starting ART at CD4 counts >500 cells/mm3 versus deferring to 350 cells/mm3 is ongoing (Strategic Timing of Anti-Retroviral Therapy).
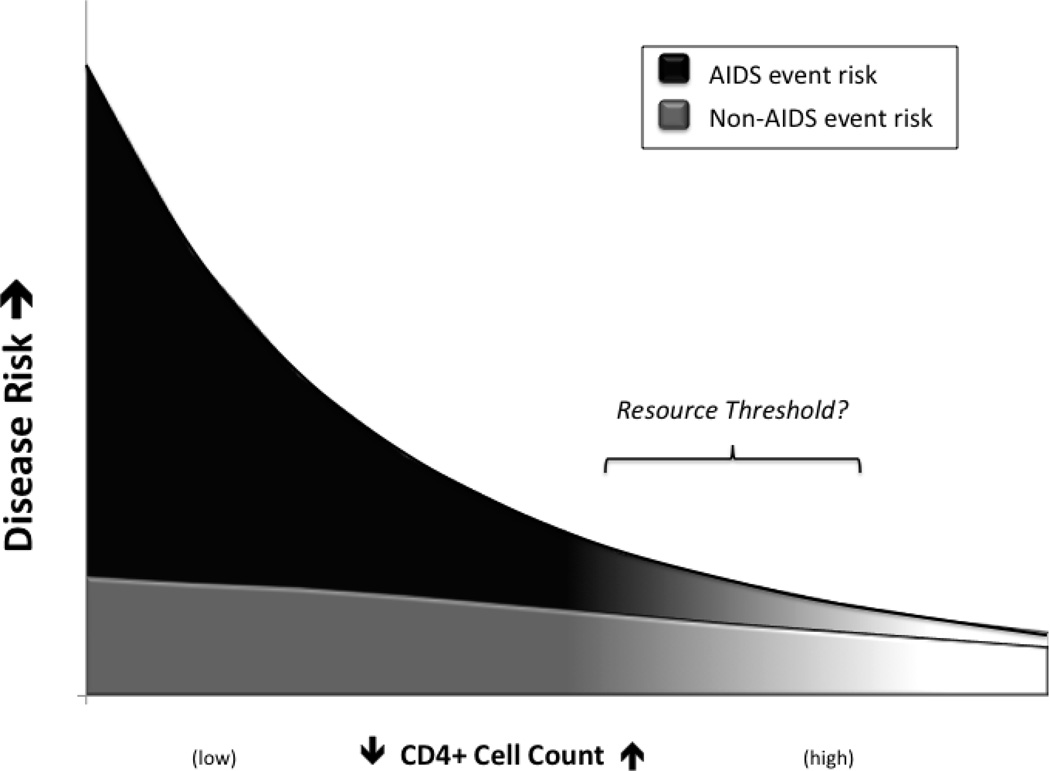
In the meantime, history would suggest we interpret the current cohort data with caution, leaving providers to have frank conversations with patients about we do and don’t know regarding starting ART. In contrast to the clear survival benefit associated with starting ART before 200 cells/mm3, the absolute clinical benefit of starting ART at higher CD4+ counts (e.g. >500 cells/mm3) will very likely be less (Figure 1). The potential for a modest benefit from starting ART in all patients must be weighed against the long-term toxicity and cost of therapy, both for the patient and society.
Figure 1. Cumulative HIV-related Disease Risk by CD4 Count.
A schematic interpretation of the benefits of ART to reduce individual health risk based on the CD4+ count. Causes of morbidity and mortality are delineated between AIDS and non-AIDS-related causes. Initiating ART at higher versus lower CD4 thresholds may result in a much smaller absolute benefit as indicated by the slope of curve. The strategy to move ART initiation earlier and at higher CD4+ counts with the ultimate goal of identifying and treating everyone will, at some point, outpace available resources.
To this end, a key aspect of efforts to expand ART use is the societal benefit from reduced transmission. The use of broad HIV testing and early treatment leads to the concept of community viral load wherein the public health goal to decrease HIV transmission is achieved by minimizing the number of persons in a community with a detectable viral load. Studies of HIV sero-discordant couples have consistently demonstrated that a lower viral load is associated with lower transmission risk (12). Examples of a similar effect of reduced HIV incidence with declines in the community viral load are being reported (13, 14). It should be noted that rates of HIV infection remain significant in all those regions, possibly driven by undiagnosed infections. Up to 50% of new HIV infections may originate from persons themselves recently infected (15), and while this supports the argument for aggressive early treatment, most of these persons are undiagnosed. Questions remain whether reduced HIV incidence with expanded ART use will continue to be sustainable over decades, and whether compensatory changes in risk behavior will attenuate or undermine this benefit (e.g, syphilis rates are currently increasing in communities reporting recent decreases HIV incidence) (16, 17).
Encouraging results of clinical studies, modeling studies, real world observations, and public health reporting lead to a sense that the HIV epidemic can be controlled by expanding indications for ART. Unfortunately, the current fiscal reality manifests that the test and treat everyone strategy requires resources beyond what appear to be available. Despite the impressive gains from wider ART access in many resource poor areas, program expansion or even maintenance is threatened by current projections for HIV funding. The President’s Emergency Program for AIDS Relief (PEPFAR) funding has leveled off, and in the U.S., nearly 7000 HIV+ patients were waiting to access ART through the AIDS Drug Assistance Program (ADAP) as of March 2011 (18). Federal and state discretionary budgets in the U.S. (including those directed at HIV care) propose severe cuts, and similar challenges will likely complicate HIV care throughout the world.
Rationing of health care, in the form of access to services, already exists in the U.S. For poor patients with HIV infection in many states, access to ART is on a first come first served basis. In real terms, there are areas in the U.S. where patients with newly diagnosed AIDS (who are at high mortality risk) wait to start ART since treatment was started earlier in the fiscal year for patients with high CD4+ counts (when the clinical benefit of ART is less). The U.S. National HIV/AIDS Strategy (19) emphasizes increasing access to care, yet that goal may be compromised by treating HIV infection on a first come, first served basis. Modeling the strategy of giving preference to patients with lower CD4+ counts yields superior outcomes (20), and this approach may need to be considered in many settings (see Figure).
Expanded use of ART has the potential to significantly curtail the future HIV epidemic. The current study is a robust and carefully performed analysis that supports the presence of a graded benefit of ART even when risk for AIDS is low, but uncertainty remains regarding the cumulative benefits in absolute terms of treating everyone with HIV infection. Investment in well-conceived clinical studies is often met with resistance given the up-front costs--particularly true in current times--but the continuing HIV epidemic and tightening resources requires we clarify the absolute benefits, risks, and costs of expanding the indications for ART. Improved care for our patients with HIV infection in an era of fiscal constraint is a goal we can achieve with sound data to inform both individual treatment and public policy decisions.
Acknowledgments
Disclosures: J. Baker receives research support from the U.S. National Institutes of Health, the U.S. Center for Disease Control and Prevention, the American Heart Association, Gilead Sciences, and ViiV Healthcare. K. Henry receives research support from the University of Minnesota/U.S. National Institutes of Health, U.S. Center for Disease Control and Prevention, Gilead Sciences, Tibotec, Glaxo-Smith-Kline, and Pfizer; is a consultant to Gilead Sciences and ViiV, and is on the Gilead Sciences and Glaxo-Smith-Kline speaker’s bureau.
References
- 1.Report of the NIH Panel to Define Principles of Therapy of HIV Infection and Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Department of Health and Human Services. MMWR. 1998;47(RR-5):1–83. [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed February 2011];Department of Health and Human Services. 2011 Jan;:1–166. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 3.The HIV-CAUSAL Collaboration. When to initiate combined antiretroviral therapy to reduce rates of mortality and AIDS-defining illness in HIV-infected individuals in developed countries. Ann Intern Med. 2011 doi: 10.1059/0003-4819-154-8-201104190-00001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.When To Start Consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CASCADE Collaboration. HAART Initiation and Clinical Outcomes: insights from the CASCADE Cohort on 'When To Start'. 2010. [Google Scholar]
- 7.Neuhaus J, Angus B, Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24(5):697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JV, Neuhaus J, Duprez D, et al. Changes in Inflammatory and Coagulation Biomarkers: A Randomized Comparison of Immediate versus Deferred Antiretroviral Therapy in Patients With HIV Infection. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 11.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 13.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 16.McCormick AW, Walensky RP, Lipsitch M, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007;44(8):1115–1122. doi: 10.1086/512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koskey A. [Accessed March 2011];Syphilis cases hit all-time high. 2011 Jan 8th; San Francisco Examiner; Available at: http://www.sfexaminer.com/local/2011/01/syphilis-cases-hit-all-time-high.
- 18.The ADAP WATCH-NASTAD. [Accessed March 2011];2011 Mar; Available at: http://www.nastad.org/InFocus/InfocusResultsDetails.aspx?infocus_id=363.
- 19.White House Office of National AIDS Policy. [Accessed March 2011];National HIV/AIDS Strategy. 2010 Jul; Available at: http://aids.gov/federal-resources/policies/national-hiv-aids-strategy/.
- 20.Linas BP, Losina E, Rockwell A, Walensky RP, Cranston K, Freedberg KA. Improving outcomes in state AIDS drug assistance programs. J Acquir Immune Defic Syndr. 2009;51(5):513–521. doi: 10.1097/QAI.0b013e3181b16d00. [DOI] [PMC free article] [PubMed] [Google Scholar]