Abstract
Despite well developed emergency medical services with rapid response advanced life support capabilities, survival rates following out-of-hospital ventricular fibrillation (VF) have remained bleak in many venues. Generally, these poor resuscitation rates are attributed to delays in the performance of basic cardiopulmonary resuscitation by bystanders or delays in defibrillation, but recent laboratory data suggest that the current standard of immediately providing a countershock as the first therapeutic intervention may be detrimental when VF is prolonged beyond several minutes. Several studies now suggest that when myocardial energy supplies begin to dwindle following more prolonged periods of VF, improvements in coronary artery perfusion must first be achieved in order to prime the heart for successful return of spontaneous circulation after defibrillation. Therefore, before countershocks, certain pharmacologic and/or mechanical interventions might take precedence during resuscitative efforts. This evolving concept has been substantiated recently by clinical studies, including a controlled clinical trial, demonstrating a significant improvement in survival when basic cardiopulmonary resuscitation is provided for several minutes before the initial countershock. Although this evolving concept differs from current standards and may pose a potential problem for automated defibrillator initiatives (e.g. public access defibrillation), successful defibrillation and return of spontaneous circulation have been rendered more predictable by evolving technologies that can score the VF waveform signal and differentiate between those who can be shocked immediately and those who should receive other interventions first.
Keywords: advanced cardiac life support, cardiac arrest, cardiopulmonary arrest, cardiopulmonary resuscitation, countershock, defibrillation, median frequency, resuscitation, scaling exponents, spectrum analysis, sudden cardiac death, ventricular fibrillation
Introduction
Sudden out-of-hospital cardiac arrest (SOHCA) remains one of the major causes of death for men and women alike in Western societies, accounting for more than 250,000 lives lost annually in the USA alone [1,2]. Ironically, most cases of SOHCA are caused by a highly reversible yet time dependent process, namely ventricular fibrillation (VF), which in turn creates a tremendous opportunity for public health intervention [1-4]. Nevertheless, despite well developed emergency medical services (EMS) with rapid response advanced cardiac life support (ACLS) capabilities, survival rates following SOHCA have remained very low in most venues, even for out-of-hospital VF [1-4].
Two key interventions have been proven scientifically to improve the chances of survival for those experiencing SOHCA: immediate performance of basic cardiopulmonary resuscitation (CPR) by bystanders; and immediate delivery of specialized countershock in cases of VF. Therefore, poor resuscitation rates in EMS systems have been attributed most often to delays in the delivery of basic CPR by witnesses or delays in rapid defibrillation by EMS personnel [3,4]. However, recent laboratory and clinical data have also begun to suggest that the current standard of immediately providing counter-shock as the first intervention for VF may be detrimental when the VF is prolonged beyond several minutes [5-9].
The mechanism underlying this is complicated and multifactorial, but, in short, several studies now suggest that when myocardial energy supplies and oxygenation begin to dwindle following prolonged periods of VF, improvements in coronary artery perfusion must first be achieved in order to prime the heart for successful return of spontaneous circulation (ROSC) after defibrillation [5-7,10-12]. Along with experimental and supportive clinical evidence, histologic and physiologic studies have resulted in an evolving hypothesis that delivery of an electrical countershock to an ischemic heart may be more damaging than when it is delivered immediately (within the first 2–3 min) following the onset of VF [13-15]. In turn, according to this paradigm, certain pharmacologic and/or mechanical interventions should take precedence over electrical countershock during resuscitative efforts if the countershocks cannot be delivered within the first few minutes following onset of VF.
Appropriate timing of advanced cardiac life support and countershocks
The evolving concept of providing 'preshock' interventions for VF may explain why several teams of investigators were not able to demonstrate the efficacy of so-called 'high dose adrenaline [epinephrine]' (i.e. >1 mg/kg doses) and other ACLS procedures in previous clinical trials when they were successful in the laboratory [16-18]. In keeping with international guidelines, these study protocols called for the use of the test intervention (e.g. high-dose adrenaline) after multiple countershocks in cases of VF [17-19]. In contrast, the successful preclinical studies used the resuscitative drugs before countershock [20]. This explanation has been substantiated by specific canine experiments conducted by Niemann and coworkers [6] that subsequently tested the resuscitation effects of high-dose adrenaline administered before and after countershocks. In such studies, ROSC was improved by first administering the high-dose adrenaline after 7.5 min of VF.
Several other animal models now strongly corroborate this concept of 'drugs first' in prolonged VF [7,12]. Using a 'cocktail' (multiple drug) regimen, including high-dose adrenaline, antiarrhythmics, and antioxidants, Menegazzi and colleagues [7] demonstrated similar effects in terms of resuscitation and short-term survival in swine that experienced 8 min of VF before interventions. Therefore, these experiments may help to explain the relative lack of effectiveness of high-dose adrenaline in clinical trials, particularly in the subset of patients presenting with VF.
In fact, in some of the clinical trials of high-dose adrenaline, on average the first drugs were given as late as 17 min following notification of the SOHCA event, even when examining cases of witnessed collapse only [16]. Many of the cities studied in the trial conducted by Brown and coworkers [17] had excellent response intervals and greater than average survival rates, thus indicating a relative 'best case' scenario. Thus, it may be speculated further that the need for preshock interventions would generally be indicated in such prolonged periods of VF, particularly when compared with animal studies demonstrating the efficacy of drugs first with much briefer periods of arrest. Therefore, it may very well be that ACLS drugs (and high-dose adrenaline in particular) may be of more value than previously demonstrated, and that their efficacies may have been masked, in part, during clinical trials because of inappropriate timing of administration relative to countershock.
Should countershocks always be delayed if ventricular fibrillation is prolonged?
If delivering countershocks first might be harmful, then should other interventions always be delivered first? Experimental studies such as those cited above appear to demonstrate the need for high-dose adrenaline and other interventions before countershock. In fact, more than two decades ago, Yakaitis and coworkers [5] showed a marked improvement in outcomes using only standard doses of adrenaline (coupled with basic CPR procedures) before countershock in a canine model. However, this preshock intervention was studied after only 5 min of VF. Therefore, it is possible that higher doses of adrenaline may only be needed after more prolonged periods of VF [20]. Nevertheless, all of these studies still indicate the need for some supportive intervention before defibrillation attempts when several minutes of untreated VF have elapsed.
More recently, preliminary clinical studies have supported this evolving concept in terms of providing basic CPR procedures (i.e. chest compressions) for a short period before defibrillation in unmonitored out-of-hospital VF [8,9]. In such scenarios, there is de facto more than several minutes of VF while the emergency response is being made, even in rapid response EMS systems. In one of these studies, conducted in the rapid response Seattle EMS system, there was still a marked improvement in outcomes when first responder firefighter crews provided 90 seconds of basic CPR before defibrillation attempts [8]. Although that study used an historical control (2 years of no preshock CPR by the first responders versus a subsequent period using 90 seconds of CPR before defibrillation attempts), survival rates were clearly improved. This finding was particularly compelling when analyzing the subset of patients who received the 90 seconds of CPR first when the EMS response intervals were greater than 4 min (Fig. 1). In the cases in which the EMS responded in less than 4 min, there was little difference in outcomes but the results were still not worse with 90 seconds of CPR first (Fig. 1).
Figure 1.
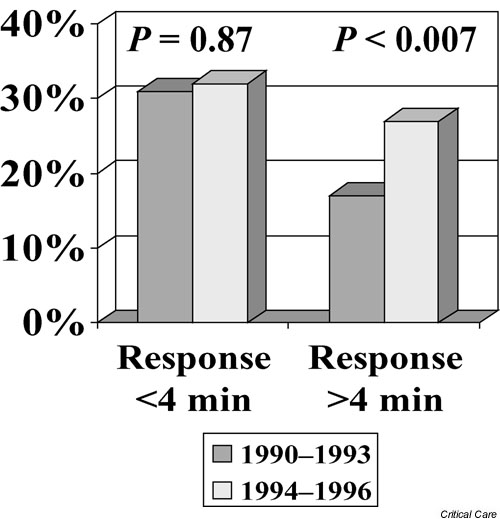
Comparison of survival rates (successful hospital discharge) in Seattle, USA, during the years when emergency responders made defibrillation attempts their first priority (1990–1993) versus subsequent years (1994–1996), when they provided 90 seconds of basic cardiopulmonary resuscitation before defibrillatory attempts for out-of-hospital cases of ventricular fibrillation. Survival rates and historical comparisons are stratified according to those patients receiving an emergency response within 4 min versus those with response intervals greater than 4 min. The response interval was measured from the time of dispatch of emergency vehicles until the time of arrival at the street address (not time of collapse to arrival at the patient's side). Adapted from Cobb and coworkers [8].
Given that this study involved a relatively short (4 min average) response interval, one might surmise that these results are good enough to support a 'intervention first' approach in all cases, especially because there seemed to be no harm in performing 90 seconds of basic CPR before shock, even in the shorter than average response periods. However, before drawing final conclusions about this study, it should be noted that even in cases of witnessed collapse there is also a finite amount of time before EMS is called after the collapse, and that there is another minute or two required to reach the patient's side and deliver the shock after on-scene arrival of EMS. Therefore, this '4 min response interval' may translate into a 7 or 8 min period of VF, and one should not immediately extrapolate a time frame for 'shock first' or 'CPR first'. In addition, one should note that basic CPR was provided by bystanders in a large percentage of these cases (in all subgroups). Therefore, many patients were already receiving some degree of basic CPR before the counter-shock, even in the historical control period.
Although the Seattle study may be subject to scrutiny because of the (historical control) study design, Wik and colleagues [9] in Oslo, Norway later reported almost identical results but in a controlled clinical trial. In their clinical trial, patients were randomly assigned to either 3 min of chest compressions first or shock first. Again, those patients receiving basic CPR first did much better, particularly in the subgroups of patients with more than 5 min EMS response intervals (i.e. presumably at least 8–9 min of VF before professional intervention). Specifically, ROSC occurred more often in the group with 3 min of CPR first when response intervals exceeded 5 min (58% versus 38%, P < 0.04) with an odds ratio of 2.22 and 95% confidence interval of 1.06–4.63. Similar to the Seattle study, ROSC was not significantly different in the groups for whom the response was less than 5 min. More impressively, survival to hospital discharge was improved (22% versus 4%, P = 0.006; odds ratio 7.42, 95% confidence interval 1.61–34.3), as was 1 year survival (20% versus 4%, P = 0.01; odds ratio 6.76, 95% confidence interval 1.42–31.4). Almost all (approximately 90%) of those discharged alive in the study were either neurologically normal or had only had minor problems (with no significant differences noted in the subgroups). Recognizing that even those patients with a response interval less than 5 min did no worse with 'CPR first' (Fig. 2), Wik and colleagues concluded that 3 min of CPR before defibrillation attempts is always indicated unless the patient collapsed in front of EMS.
Figure 2.
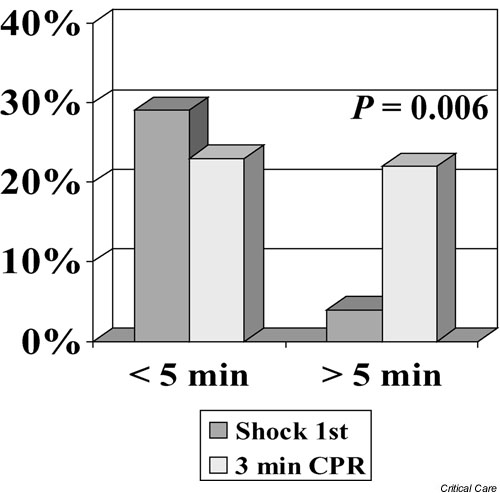
Comparison of out-of-hospital ventricular fibrillation survival rates (successful hospital discharge) with defibrillation attempts provided first versus cases for which there was provision of 3 min of basic cardiopulmonary resuscitation before defibrillation attempts in Oslo, Norway. Survival rate comparisons are stratified according to those patients receiving an emergency response within 5 min versus those with response intervals greater than 5 min. The response interval was measured from the time of dispatch of emergency vehicles until the time of arrival at the street address (not time of collapse to arrival at the patient's side). Adapted from Wik and coworkers [9].
The strength of these clinical data has added more credibility to the evolving notion that interventions should always be performed before defibrillation attempts. However, this proposed approach does pose problems for current resuscitation policies. In addition to conflicting with internationally accepted standards of patient management [19], the deferred counter-shock concept may also pose a potential glitch for current automated defibrillator initiatives, especially certain public access defibrillation initiatives [21,22]. In addition, successful defibrillation and ROSC can be achieved after relatively prolonged periods of arrest especially with well performed and immediately executed basic CPR [3,4]. In most cases of successful resuscitation from VF, resuscitative drugs are never needed, even after the countershock [4,16]. Therefore, one must interpret the evolving evidence for interventions before countershock within context. If the heart remains well perfused, then the shock may still be delivered first.
Furthermore, in the early canine experiments conducted by Yakaitis and coworkers [5] that demonstrated the superiority of giving adrenaline and CPR before countershocks after 5 min of VF, companion experiments also demonstrated that shocking first was clearly superior following only 1 min of VF. Also, recent studies have indicated very high survival rates when patients are shocked within 5 min, such as a recent study of public access defibrillation at the Chicago municipal airports. In that study of public use of automated defibrillators, three quarters of the patients were resuscitated and achieved full neurologic recovery when shocked within 5 min of collapse. In fact, many of the patients were already awakening by the time of EMS arrival at the scene. Nevertheless, the authors also noted that all survivors received some period of chest compressions and other basic CPR techniques, even if briefly, while awaiting defibrillatory attempts.
Assimilating all of the studies to date, one might conclude that rapid defibrillation should be a priority in the first few minutes after arrest, but that basic CPR may also be provided as long as it does not delay the defibrillatory attempts. However, after several minutes of arrest (perhaps 4 or 5 min), basic CPR and perhaps other ACLS interventions may need to be provided for a finite period of time before the shocks.
It is clear, however, that such judgments and time determinants are all guesswork and that many factors, particularly the rapid provision of well performed early basic CPR, may be confounding variables. Therefore, somehow being able to delineate objectively between a hypoxic and nonhypoxic heart might be a critical adjunct to therapeutic decisions.
Objective guides for defining the priority of interventions
In addition to defining whether defibrillation should be deferred, it would also be important to define what therapies are required at any given point, be they chest compressions alone, chest compressions and adrenaline, high-dose adrenaline and other drugs, or perhaps new alternative CPR devices. In addition, it remains unclear as to whether chest compressions alone are indicated after a few minutes of VF or whether drug infusions should also be given. Likewise, it may turn out that, at some point in the protraction of VF, multiple drugs or progressively higher doses of drugs may be needed before countershock. Again, all of these considerations must be addressed within the context of a number of confounding variables such as scenarios involving immediate and well performed bystander CPR or scenarios of chest pain or ventricular tachycardia (with spontaneous pulses) deteriorating into VF just before arrival of the rescuers with a defibrillator. Therefore, having the technology or ability to predict the level of ischemia in the heart would be more useful than a stopwatch.
Fortunately, successful defibrillation with ROSC following a countershock first approach may be more predictable with real-time scoring of the VF waveform signal. Specifically, techniques such as online electrocardiographic median frequency or scaling exponent analysis can be used to predict successful defibrillation [12,23-27]. Conceptually, in a real-time setting, a defibrillator can perform an analysis of the VF waveform and score the electrical signal. If the score is high enough (or low enough, depending on the analysis), then a shock would be advised. If missing the mark, other therapies would be advised first and perhaps at progressively different levels depending on the severity of the poor score. Studies have shown, for example, that basic CPR and certain pharmacologic interventions can (but not always) improve the VF waveform score [12,24-26]. Therefore, one might speculate that, in the future, user-friendly technology with automated algorithms will be developed that will not only guide the type and degree of initial therapeutic interventions, but also the duration of resuscitative efforts. Moreover, such technology will help us to better define different phases of resuscitative therapies [28].
Conclusion
It has become clear that the timing of certain interventions in SOHCA is time dependent or, more accurately, dependent upon the duration and degree of the ischemic insult after the onset of VF. Although the overall concept of providing certain therapeutic interventions before countershock in cases of prolonged VF has become very compelling, it must always be appreciated that there are multiple variables that may confound the appropriateness of this approach. Although new technologies may eventually help to overcome these concerns, the dynamics of proposed waveform analyses and their specific relationships to successful ROSC and ultimate outcome must be carefully weighed against the clinical circumstances. In addition, there are also factors related to the intervention used, such as the type of countershock being delivered. For example, low-energy biphasic shocks and other evolving energy delivery mechanisms may behave differently than traditional high-energy or monophasic shocks [12,29-31]. There are also new CPR devices that may be found to be more effective than current techniques in providing resuscitation after countershock delivery [28].
Nevertheless, the evolving evidence for preshock therapies following several minutes of VF is very strong. Although it will require aggressive, multifaceted studies to delineate the many confounding variables and the specific interventions that should be delivered under specific circumstances, the preliminary data certainly justify further study. Interestingly, in many ways, these data revalidate the important discovery of basic CPR more than four decades ago. In addition, today, with the introduction of various promising resuscitative techniques such as the active compression–decompression pump, 'vest' CPR, the inspiratory threshold device and minimally invasive direct cardiac massage, it is plausible that we may be able to resuscitate many more persons than ever before, particularly if these interventions are applied before defibrillation attempts [32].
Competing interests
None declared.
Abbreviations
ACLS = advanced cardiac life support; CPR = cardiopulmonary resuscitation; EMS = emergency medical services; ROSC = return of spontaneous circulation; SOHCA = sudden out-of-hospital cardiac arrest; VF = ventricular fibrillation.
See related Commentary: http://ccforum.com/content/8/1/11
References
- Zheng ZJ, Croft JB, Giles WH, Mensah GH. Specific mortality from sudden cardiac death: United States 1999. MMWR Morb Mortal Wkly Rep. 2002;51:123–126. [PubMed] [Google Scholar]
- American Heart Association . American Heart Association 2002 Heart and Stroke Statistical Update. Dallas, TX: American Heart Association; 2002. pp. 1–13. [Google Scholar]
- Cummins RO, Ornato JP, Thies W, Pepe PE. Improving survival from sudden cardiac arrest: the 'chain of survival' concept. Circulation. 1991;83:1832–1847. doi: 10.1161/01.cir.83.5.1832. [DOI] [PubMed] [Google Scholar]
- Becker LB, Pepe PE. Ensuring the effectiveness of community-wide emergency cardiac care. Ann Emerg Med. 1993;22:354–365. doi: 10.1016/s0196-0644(05)80465-2. [DOI] [PubMed] [Google Scholar]
- Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8:157–163. doi: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Cairns CB, Sharnia J, Lewis RJ. Treatment of prolonged ventricular fibrillation: immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation. 1992;85:281–287. doi: 10.1161/01.cir.85.1.281. [DOI] [PubMed] [Google Scholar]
- Menegazzi JJ, Seaberg DC, Yealy DM, Davis EA, MacLeod BA. Combination pharmacotherapy with delayed countershock versus standard advanced cardiac life support after prolonged ventricular fibrillation. Prehosp Emerg Care. 2000;4:31–37. doi: 10.1080/10903120090941614. [DOI] [PubMed] [Google Scholar]
- Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- Wik L, Hansen TB, Fylling F, Vaagenes P, Auestad BH, Steen PA. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular defibrillation: a randomized trial. JAMA. 2003;289:1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- Kern KB, Garewal HS, Sanders AB. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation. 1990;20:221–229. doi: 10.1016/0300-9572(90)90005-Y. [DOI] [PubMed] [Google Scholar]
- Ditchey RV, Goto Y, Lindenfeld J. Myocardial oxygen requirements during experimental cardiopulmonary resuscitation. Cardiovasc Res. 1992;26:791–797. doi: 10.1093/cvr/26.8.791. [DOI] [PubMed] [Google Scholar]
- Angelos MG, Menegazzi JJ, Callaway CW. Bench to bedside: resuscitation from prolonged ventricular fibrillation. Acad Emerg Med. 2001;8:909–924. doi: 10.1111/j.1553-2712.2001.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Gaba DM, Talner NS. Myocardial damage following transthoracic direct current countershock in newborn piglets. Fed CardioI. 1982;2:281–288. doi: 10.1007/BF02426974. [DOI] [PubMed] [Google Scholar]
- Tacker WA, Van Fleet JF, Geddes LA. Electrocardiographic and serum enzymatic alterations associated with cardiac alterations induced in dogs by single transthoracic damped sinusoidal defibrillation shocks of various strengths. Am Heart J. 1979;98:85–193. doi: 10.1016/0002-8703(79)90220-5. [DOI] [PubMed] [Google Scholar]
- AI-Khadra A, Nikolski V, Efimov IR. The role of electroporation in defibrillation. Circ Res. 2000;87:797–804. doi: 10.1161/01.res.87.9.797. [DOI] [PubMed] [Google Scholar]
- Pepe PE, Abramson NS, Brown CG. ACLS: does it really work? Ann Emerg Med. 1994;23:1037–1041. doi: 10.1016/s0196-0644(94)70100-8. [DOI] [PubMed] [Google Scholar]
- Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, Jastremski M. Standard versus high-dose epinephrine in out-of-hospital cardiac arrest: a controlled clinical trial. N Engl J Med. 1992;327:1051–1055. doi: 10.1056/NEJM199210083271503. [DOI] [PubMed] [Google Scholar]
- Callaham M, Madsen CD, Barton CW, Sounders CE, Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA. 1992;268:2667–2672. doi: 10.1001/jama.268.19.2667. [DOI] [PubMed] [Google Scholar]
- Anonymous 2000 Guidelines for cardiopulmonary resuscitation and emergency cardiac care [special supplement] Circulation. 2000;102:1–384. [Google Scholar]
- Brown CG, Werman HA, Davis EA, Hobson J, Hamlin RL. The effects graded doses of epinephrine on regional myocardial blood flow during cardiopulmonary resuscitation in swine. Circulation. 1987;75:491–497. doi: 10.1161/01.cir.75.2.491. [DOI] [PubMed] [Google Scholar]
- Weisfeldt ML, Kerber RE, McGoldrick RP, Moss AJ, Nichol J, Ornato JP, Palmer DG, Riegel B, Smith SC, for the Automatic External Defibrillation Task Force American Heart Association report on the Public Access Defibrillation Conference, December 8–10, 1994. Circulation. 1995;92:2740–2747. doi: 10.1161/01.cir.92.9.2740. [DOI] [PubMed] [Google Scholar]
- Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. N Engl J Med. 2002;347:1242–1247. doi: 10.1056/NEJMoa020932. [DOI] [PubMed] [Google Scholar]
- Brown CG, Dzwonczyk R. Signal analysis of the human electrocardiogram during ventricular fibrillation: frequency and amplitude parameters as predictors of successful counter-shock. Ann Emerg Med. 1996;27:184–188. doi: 10.1016/s0196-0644(96)70346-3. [DOI] [PubMed] [Google Scholar]
- Strohmenger HU, Linder KH, Crown CG. Analysis of the ventricular fibrillation ECG signal amplitude and frequency parameters as predictors of countershock success in humans. Chest. 1997;111:584–589. doi: 10.1378/chest.111.3.584. [DOI] [PubMed] [Google Scholar]
- Noc M, Weil MH, Gazmuri RJ, Sun S, Biscara J, Tang W. Ventricular fibrillation voltage as a monitor of the effectiveness of cardiopulmonary resuscitation. J Lab Clin Med. 1994;124:421–426. [PubMed] [Google Scholar]
- Strohmenger HU, Linder KH, Keller A, Linder IM, Pfenninger E, Bothner U. Effects of graded doses of vasopressin on median fibrillation frequency in a porcine model of cardiopulmonary resuscitation: results of a prospective, randomized, controlled trial. Crit Care Med. 1996;24:1360–1365. doi: 10.1097/00003246-199608000-00015. [DOI] [PubMed] [Google Scholar]
- Weaver WD, Cobb LA, Dennis D. Amplitude of ventricular fibrillation waveform and outcome after cardiac arrest. Ann Intern Med. 1985;8:157–163. doi: 10.7326/0003-4819-102-1-53. [DOI] [PubMed] [Google Scholar]
- Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- Cummins RO, Hazinski MF, Kerber RE, Kudenchuk P, Becker L, Nichol G, Malanga B, Aufderheide TP, Stapleton EM, Kern K, Ornato JP, Sanders A, Valenzuela T, Eisenberg M. Low-energy biphasic waveform defibrillation evidence-based review applied to emergency cardiovascular care guidelines. Circulation. 1998;97:1654–1667. doi: 10.1161/01.cir.97.16.1654. [DOI] [PubMed] [Google Scholar]
- Wang HE, Menegazzi JJ, Lightfoot CB, Callaway CW, Fertig KC, Sherman LD, Hsieh M. Effects of biphasic versus monophasic defibrillation on the scaling exponent in a swine model of prolonged ventricular fibrillation. Acad Emerg Med. 2001;8:771–780. doi: 10.1111/j.1553-2712.2001.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Ivey TD, Allen MD, Johnson G, Mehra R, Greene HL. A prospective randomized evaluation of biphasic versus monophasic waveform pulses on defibrillation efficacy in humans. J Am ColI Cardiol. 1989;14:728–733. doi: 10.1016/0735-1097(89)90118-6. [DOI] [PubMed] [Google Scholar]
- O'Connor RE, Ornato JP, Wigginton JG, Hunt RC, Mears G, Penner J. Alternative cardiopulmonary resuscitation devices. Prehosp Emerg Care. 2003;7:31–41. doi: 10.1080/10903120390937067. [DOI] [PubMed] [Google Scholar]


