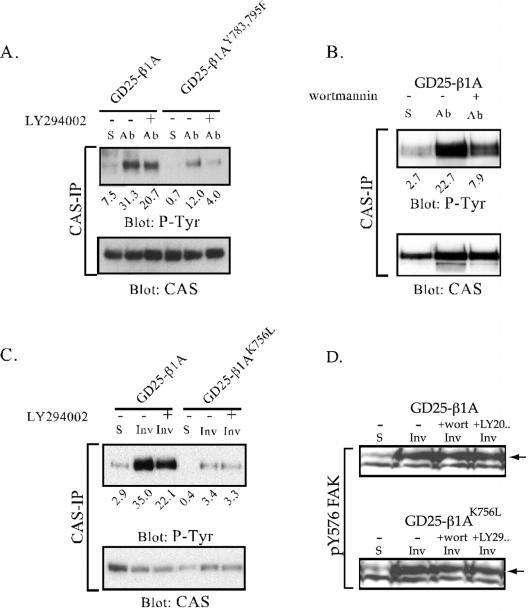
Figure 9.
Tyrosine phosphorylation of CAS and FAK after β1-mediated adhesion in the presence of PI3K inhibitors. CAS was immunoprecipitated from cell lysates prepared from suspended cells (S) or from cells adhering to anti-β1 mAb (Ab) or to invasin (Inv) for 1 h. For the analysis of FAK phosphorylation the cells were lysed directly in SDS sample buffer. Before plating, the cells were treated with LY294002 (20 μM; A and C) or wortmannin (100 nM; B) (+), or with the diluent (dimethyl sulfoxide) as a control (-). Precipitated material was subjected to SDS-PAGE, transferred to nitrocellulose filters, and sequentially blotted for phosphotyrosine and CAS protein. The relative intensities of the bands versus the gel background are represented by arbitrary units under each lane. In D, equal amounts of cell lysate were blotted for pY576 FAK. Arrows point at bands corresponding to FAK.