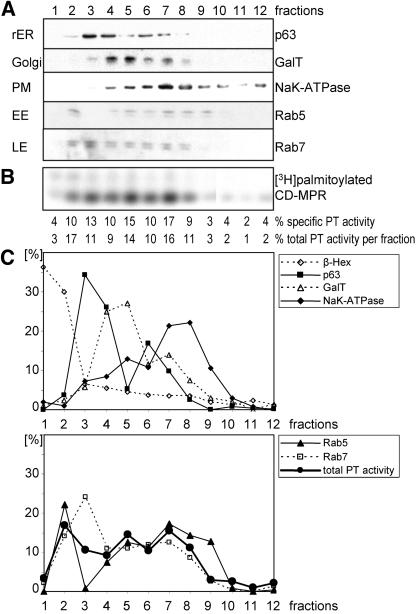
Figure 3.
HeLa cell fractionation assayed for palmitoyltransferase (PT) activity in vitro. (A) Postnuclear supernatant of HeLa cells was separated on a 15% Percoll density gradient. Fractions were collected from bottom (fraction 1) to top (fraction 12) of the gradient. Membranes of fractions were centrifuged, and pellets were resuspended in equal volumes. Equal volumes of fractions were tested for expression of marker enzymes of the different organelles by immunoblotting with the respective antibodies: galactosyltransferase (GalT) for Golgi, NaK-ATPase for plasma membrane (PM), p63 for rough endoplasmic reticulum (rER), Rab7 for late endosomes (LE), and Rab5 for early endosomes (EE). (B) One hundred fifty micrograms of each fraction was assayed for palmitoyltransferase activity in vitro. The samples were incubated with [3H]palmitoyl-CoA and purified CD-MPR at 37°C for 30 min. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography. The percentage of specific activity in each fraction was quantitated by densitometric scanning. The total activity per fraction was calculated using the protein concentration of each fraction. (C) Two graphs with the percentages of marker enzymes per fraction and the total palmitoyltransferase activity per fraction. Immunoblot shown in A was quantitated by densitometric scanning. Levels of β-hexosaminidase, a lysosomal marker enzyme, were measured with an activity assay with equal volumes of fractions.