Abstract
Mast cells (MCs) have been thought to play a pathogenic role in the development of autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE), an animal model of MS. However, an immunoregulatory function of these cells has been recently suggested. We investigated the role of MCs in EAE using the W-sh mouse strain, which is MC deficient. W-sh mice developed earlier and more severe clinical and pathological disease, with extensive demyelination and inflammation in the CNS. The inflammatory cells were mainly comprised of CD4+ T cells, monocyte/macrophages, neutrophils and dendritic cells (DCs). Compared to wild-type mice, MC-deficient mice exhibited an increased level of MCP-1/CCR2, and CD44 expression on CD4+ T cells, in addition to decreased production of Treg, IL-4, IL-5, IL-27 and IL-10. We also found that levels of IL-17, IFN-γ, and GM-CSF were significantly increased in peripheral lymphocytes from immunized W-sh mice compared with wild-type mice. Reconstitution of W-sh mice downregulated susceptibility to EAE, which correlated with MC recruitment and Treg cells activation in the CNS. These findings indicate that responsiveness is not required in the pathogenesis of inflammatory demyelination in the CNS, and that, in the absence of MCs, increased MCP-1, CCR2, IL-17, IFN-γ, CD44 and other inflammatory molecules may be responsible for increased severity of EAE.
Keywords: Experimental autoimmune encephalomyelitis (EAE), Demyelination, CNS, mast cells
Introduction
Experimental autoimmune encephalomyelitis (EAE) is a CD4+ T cell-mediated demyelinating autoimmune disease of the central nervous system (CNS) in mice (1), which serves as a model of multiple sclerosis (MS) in humans. EAE in rodents is characterized by histological lesions in the CNS, typically consisting of demyelination and infiltrates of autoreactive T cells and other mononuclear cells (1). Autoreactive T cells directed against myelin antigens produce high levels of proinflammatory cytokines such as IFN-γ, IL-17, while resistance to, or recovery from, the disease is mediated through the immunoregulatory cytokines IL-4, IL-5, IL-10, and IL-27, among others (2).
Mast cells (MCs) are derived from haematopoietic stem cells, which migrate into vascularized tissues and serosal cavities where they complete their maturation (3). Although these cells reside predominantly in tissue exposed to the external environment, including the skin, intestinal tract, and trachea, they are also normally present in the heart, lymph nodes, spleen, and the CNS (4, 5). The heterogeneity of these cells is suggested by their ability to produce an array of both pro- and anti-inflammatory mediators and act as antigen-presenting cells expressing a spectrum of co-stimulatory molecules (6). MCs and T cells can influence one another in their function (7). MCs can act as antigen- presenting cells (APC), expressing a spectrum of co-stimulatory molecules, including CD80, CD86, CD153, 4-1BB, CD40L, and OX40L (6). Purified populations of MCs can present Ags to T cells by either MHC I- or class II-restricted mechanisms (8, 9). Inducible MHC class II expression by MCs supports effector and regulatory T cell activation. Most importantly, MCs process and present Ags directly to T cells, with preferential expansion of Ag-specific regulatory T cells over other T cells (10). Activated MCs, Tregs, and Th17 cells display tight spatial interactions (11, 12). Thus, MCs are far more functionally diverse than previously thought and might act as immunoregulatory cells that influence both innate and adaptive immunity in autoimmune disease (4, 6, 13, 14).
Interestingly, recent studies have shown an immunoregulatory property for MCs in an allo-transplantation system (15), autoimmune arthritis (16) and inflammatory bowel disease (17). MCs have been suggested to be crucial to the development of EAE, given that a mast cell-deficient mouse strain, WBB6F1-Kitw /Kitw-v(W/W-v), is resistant to this disease (18, 19). However, other earlier reports showed that MC stabilizers did not ultimately alter severity of the disease (20-22) and another study reported that MC accumulation in the CNS during inflammation is dispensable for EAE (23). These results suggest that MCs have far greater pleiotropic effects in CNS disease than previously thought, but the role of MCs in the pathogenesis of MS/EAE still remains unclear.
In the present study we investigated the role of MCs in EAE using W-sh mice. We report the novel finding of early onset and more severe EAE in W-sh mice, with extensive inflammation and demyelination in the CNS. Potential mechanisms underlying this observation are further investigated.
Materials and Methods
Mice and EAE induction
Eight- to ten-week-old female C57BL/6, KitW-sh/KitW-sh (W-sh) mice and their wild-type controls were purchased from the Jackson Laboratory (Bar Harbor, ME). To induce EAE, mice were injected s.c. with/without 200 μg of myelin oligodendrocyte protein (MOG)35-55, in CFA containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) at two sites on the back. Two hundred ng of pertussis toxin was given i.v. on days 0 and 2 post immunization (p.i.). EAE was scored according to a 0∼5 scale as follows (24): 1, limp tail or waddling gait with tail tonicity; 2, waddling gait with limp tail (ataxia); 2.5, ataxia with partial limb paralysis; 3, full paralysis of one limb; 3.5, full paralysis of one limb with partial paralysis of second limb; 4, full paralysis of two limbs; 4.5, moribund; and 5, death. All work was performed in accordance with the guidelines for animal use and care at Thomas Jefferson University.
Histopathology
On day 18 p.i., mice were extensively perfused, and spinal cords were harvested. Five-micrometer sections were stained with H&E or Luxol fast blue (myelin stain). Slides were assessed in a blinded fashion for inflammation and demyelination (25). For inflammation, the following scale was used: 0, none; 1, a few inflammatory cells; 2, organization of perivascular infiltrates; and 3, increasing severity of perivascular cuffing with extension into the adjacent tissue. For demyelination, the following scale was used: 0, none; 1, rare foci; 2, a few areas of demyelination; and 3, large (confluent) areas of demyelination.
Intracellular cytokine staining and FACS analysis
MNCs from the spinal cords and brain were isolated as previously described (25). Pooled cells were washed in FACS buffer. After blocking with CD16/CD32 mAb, cells were incubated with Abs to murine CD3, CD4, CD8, CD11b, CD11c, CD45, CD44, Gr-1 and CD11a (all from BD PharMingen, San Jose, CA). To determine T cell phenotype (Th1, Th2, Th17, and Treg), CD4+ T cells that produced IFN-γ, IL-4 and IL-17 and expressed Foxp3 were analyzed by flow cytometry. MNCs were stimulated with 15 μg/ml MOG35-55 peptide for 72 h and restimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 750 ng/ml ionomycin for the last 4 h in the presence of 10 μg/ml Brefeldin A. Intracellular cytokine staining (26) and Foxp3 staining were performed as described (25, 27). Cells were analyzed by FACSAria flow cytometer (Becton Dickinson), and data obtained were analyzed by FlowJo software (Tree Star).
Cytokine production and proliferation assay
Suspensions of MNCs from the spleen were prepared on day 10 p.i. Cells were cultured at a density of 2.5 × 106/ml in medium containing MOG35-55 at final concentrations of 10 μg/ml, Con A at 5 μg/ml, or without Ag/mitogen. Supernatants were collected after 48 h. Quantitative ELISA for IFN-γ, GM-CSF, IL-4, IL-5, IL-10, IL-17 and MCP-1 was performed using paired mAbs according to the manufacturer's recommendation (BD PharMingen). To analyze MCP-1 production, spinal cord and supernatants of homogenized spinal cords were prepared as described (28) and MCP-1 levels were quantified using ELISA. To determine IL-17A, IL-27p28 and CCR2 mRNA expression, freshly isolated splenocytes were assayed using real-time PCR, with β-actin expression serving as control. Relative expression was calculated following the previously described protocol (25).
For proliferation, cells were cultured in triplicate with MOG35-55 (25 μg/ml), Con A (5 μg/ml), or without Ag/mitogen. After 60 h of incubation, cells were pulsed for 12 h with 1 μCi of [3H] methylthymidine, harvested, and [3H]-thymidine incorporation (cpm) was read using a beta counter. The results were expressed as stimulation index, which was calculated by dividing the cpm from culture in the presence of Ag or mitogen by the cpm from culture without Ag/mitogen.
Transfer of bone marrow-derived mast cells (BMMC) in W-sh mice
To prepare BMMCs, bone marrow cells from WT mice were differentiated for 5 weeks in the presence of mouse IL-3 and stem cell factor (both from PeproTech, NJ) as previously described (29). The purity of MCs was 98.7% as determined by CD117+FcεR-1α+ expression using flow cytometry. To observe the effect on EAE of transferring MC in mast-deficient mice, MCs (5× 106 per mouse) were injected by tail vein injection to W-sh mice before onset of EAE. Clinical signs were scored according to a 0∼5 scale as described (24).
Statistics
Clinical scores were analyzed using the Mann-Whitney U test, and all other experiments were tested for statistical differences using unpaired, 2-tailed, Student's t tests. Differences were considered significant if p<0.05.
Results
Increased susceptibility to EAE in mast cell-deficient W-sh mice
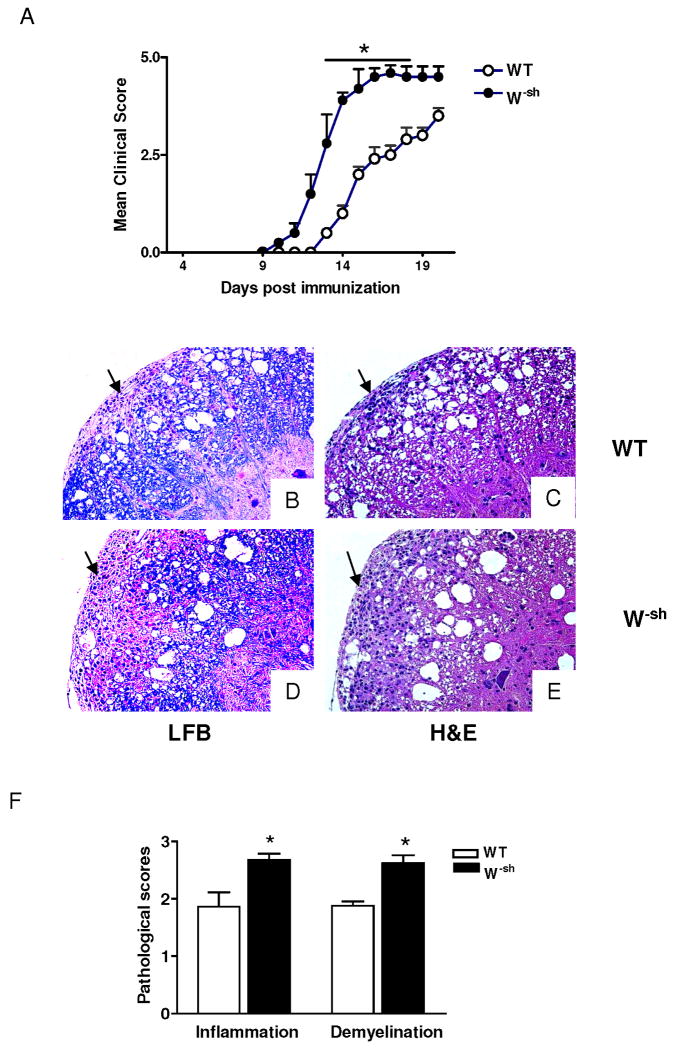
To investigate the role of MCs in the pathogenesis of EAE, we immunized W-sh mice and their wild-type controls with MOG35-55 peptide in CFA. Chronic progressive EAE was observed in immunized wild-type mice. No mice from this group (n =15) died or were moribund during the time of observation. In contrast, W-sh mice developed earlier and more severe EAE (Fig. 1A). Two of 13 mice in the W-sh group were moribund at day 17 p.i. The difference in both time of EAE onset and clinical scores between the two groups was significant (both p< 0.01). However, W-sh mice injected with the mixture CFA, pertussis toxin, Mycobacterium tuberculosis H37Ra, but omitting MOG35-55, did not develop EAE (data not shown).
Fig. 1. W-sh mice develop more severe EAE.
Female wild-type and W-sh mice (n = 5 in each group) were immunized with 200 μg of MOG35-55 peptide in CFA. (A) Clinical EAE was scored daily according to a 0∼5 severity scale. Data represent the mean clinical score ± SD. The overall clinical score was significantly different between wild-type and W-sh mice (**, p<0.01). One representative experiment of two is shown (total n =15 wild-type and 13 W-sh mice). Spinal cord histology staining. Wild-type mice (B, C) and W-sh mice (D, E) were immunized with MOG35-55 in CFA and sacrificed at day 18 p.i. Spinal cords were harvested after extensive perfusion, and 5-μm sections were stained with H&E or Luxol fast blue (myelin stain). Demyelinating lesions and inflammatory cell infiltration foci showed in the white matter (arrows). (F) Mean values and SD of spinal cord histology (n= 5 each group). *, p<0.05. One representative experiment of three is shown.
Severe inflammatory demyelination characterizes EAE in W-sh mice
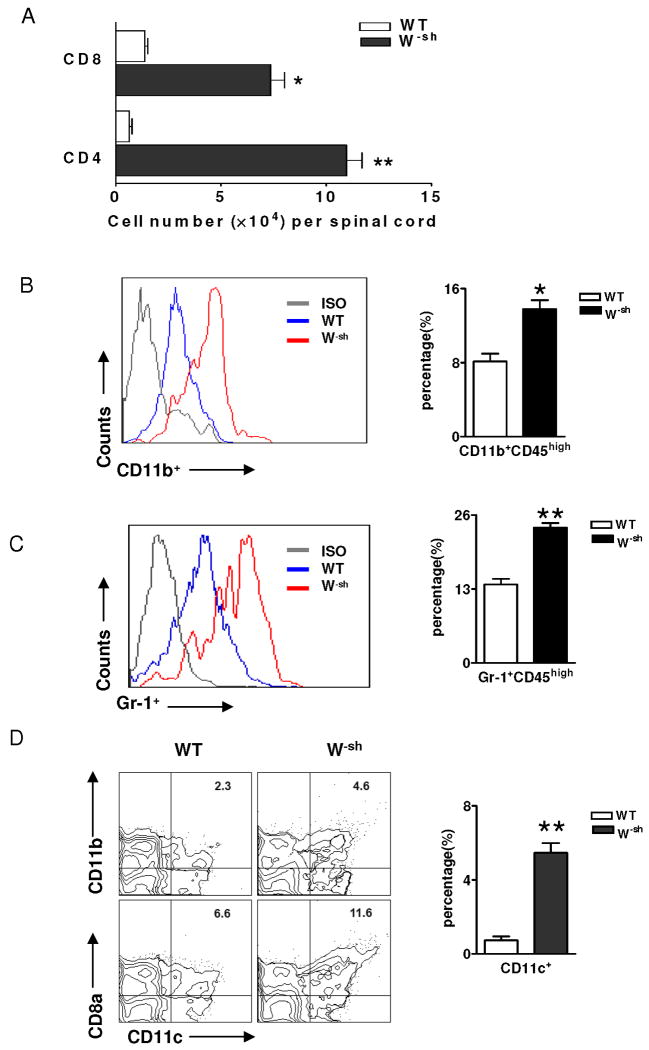
Consistent with clinical signs, typical MNC infiltration and demyelination foci were observed in the white matter of the spinal cord of both wild-type (Fig. 1B,C) and W-sh mice (Fig. 1D,E). Inflammation scores were 1.8 ± 0.3 in wild-type mice vs. 2.7 ± 0.1 in W-sh mice (p<0.05) and demyelination scores were 1.6 ± 0.1 vs. 2.6 ± 0.2 (p<0.05; Fig. 1F). More severe inflammatory infiltration in W-sh mice was further demonstrated by the significantly increased total number of CNS-derived MNCs (2.8 × 105/spinal cord vs. 1.1 ×105 in wild-type mice). These MNCs included increased CD4+ and CD8+ T cells (Fig. 2A), macrophages (CD11b+CD45hi, 22.4% in W-sh vs. 7.9% in wild-type mice; Fig. 2B), and neutrophils (Gr-1+CD45hi Fig. 2C). The numbers of CNS DCs, which included both CD8α+CD11c+ and CD11b+CD11c+ cells, were increased in W-sh mice compared to wild-type mice (Fig. 2D). Thus, a clear correlation was found between clinical and pathological features of EAE in wild-type and W-sh mice.
Fig. 2. Pattern of cellular infiltrates in spinal cords of wild-type and W-sh mice.
(A) Flow cytometric analysis. On day 18 p.i., mice were extensively perfused, and spinal cords were harvested and pooled for each mouse group (n = 4 in wild-type mice and n = 5 in W-sh mice). Total numbers of CD4+ and CD8+ T cells were calculated by multiplying the percentage of total cell numbers in spinal cord. For infiltrating macrophages (CD11b+CD45hi) and neutrophils (Gr-1+CD45hi), CD45hi cells were gated and their expression of CD11b (B) and Gr-1 (C) were determined. (D) Percentages of CD11b+ CD11c+ and CD8α+ CD11c+ DC subsets in spinal cord of wild type and W-sh mice. *, p<0.05; **, p<0.01. One representative experiment of two is shown.
Increased proliferative responses to autoantigen and production of proinflammatory cytokines in W-sh mice
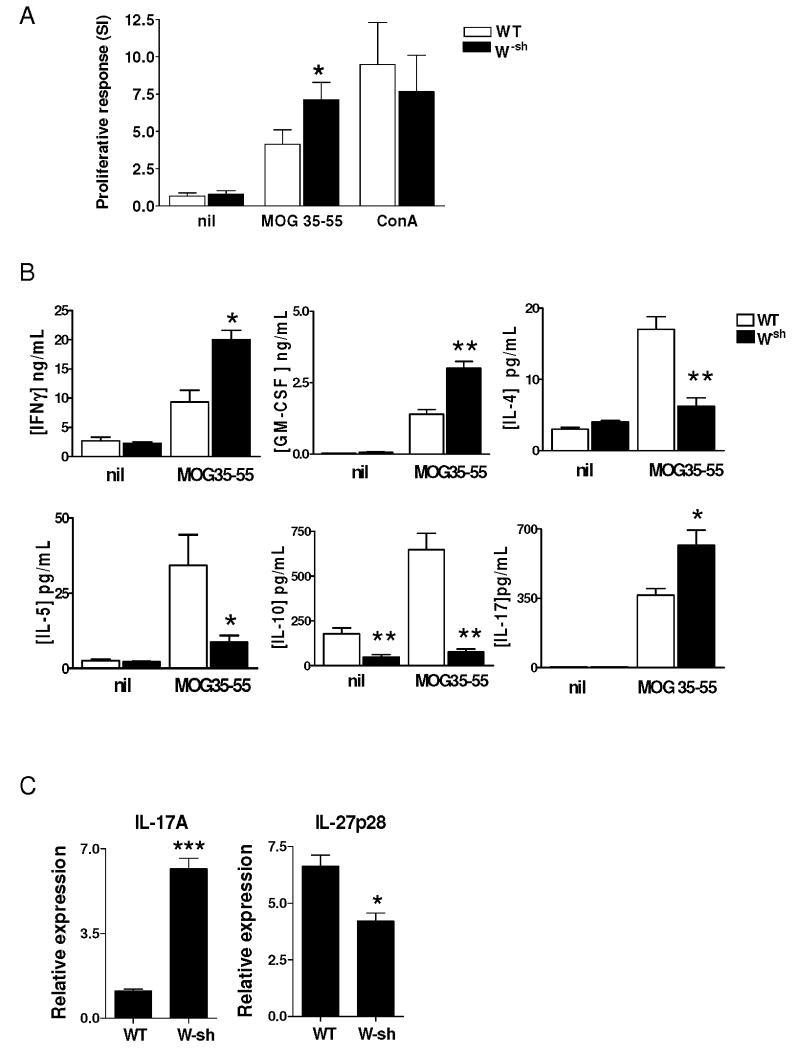
At day 10 p.i., a MOG-specific proinflammatory response was observed in spleen-derived lymphocytes from wild type mice. This response was characterized by vigorous MOG-induced proliferation and production of inflammatory cytokines. In W-sh mice, which developed more severe EAE, a significantly higher proinflammatory response was observed compared to wild type mice, including increased proliferation of specific T cells to MOG (p<0.05; Fig. 3A), high levels of proinflammatory cytokines IFN-γ, GM-CSF and IL-17 (Fig. 3B), and increased IL-17 mRNA expression (6-fold higher in W-sh mice than in wild-type mice; Fig. 3C). In contrast, W-sh mice exhibited significantly decreased production of Th2 cytokines IL-4 and IL-5 (both p<0.05), and IL-10 (p<0.001) (Fig. 3B). Expression of the p28 subunit of IL-27 was also significantly lower in W-sh mice than in wild type mice (p<0.05; Fig. 3C). Thus, W-sh mice exhibited a bias toward proinflammatory response to autoantigen.
Fig. 3. Autoantigen-induced proliferative response and cytokine production.
Pooled splenocytes (4 ×105/well) from wild-type (n = 4) and W-sh mice (n = 5; day 10 p.i.) were cultured with MOG35-55 at 10 μg/ml, Con A at 5 μg/ml, or without Ag/mitogen for proliferative responses (A). (B) Production of cytokines was determined from 48 h culture supernatants by ELISA. (C) mRNA expression of IL-17A and IL-27p28 in uncultured splenocytes was determined by RT-PCR. Data represent mean values and SD of three separate experiments. P values refer to comparison between two groups. *, p<0.05; **, p<0.01 and ***, p<0.001.
Th17 vs. Treg responses in W-sh mice
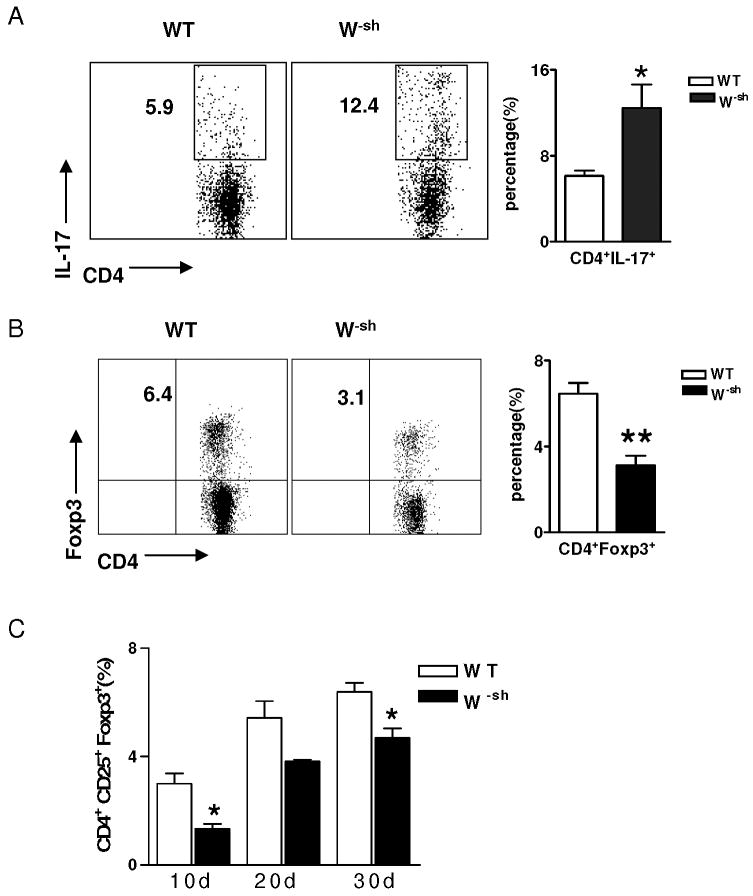
By intracellular staining, we found that the percentage of Th17 cells (CD4+IL-17+) in the CNS was significantly increased in W-sh mice compared to wild-type mice (12.4 ± 2.2 vs. 5.9 ± 0.5; p<0.05); (Fig. 4A). In contrast, the CD4+Foxp3+ population in the CNS was decreased from 6.4% in wild-type mice to 3.1% in W-sh mice (Fig. 4B). The percentage of Foxp3+ cells in gated CD4+ T cells was analyzed at different time points of EAE (Fig. 4C). We concluded that enhanced severity of EAE in W-sh mice is associated with increased responses of self-reactive T cells and lower proportions of regulatory T cells.
Fig. 4. Th17 vs. Treg responses in W-sh mice.
(A) Inflammatory cells were isolated from pooled spinal cords of wild-type (n =4) and W-sh mice (n = 5; day 18 p.i.) and were cultured for 48 h and stimulated with PMA/ionomycin in the presence of Brefeldin A (10 μg/ml) for the last 4 hrs of culture prior to staining with fluorescently labeled antibodies. Lymphocytes were gated, and IL-17 expression on CD4+ T cells of wild-type and W-sh mice (n = 5 in each group) was determined and percentages of IL-17+CD4+ T cells in spinal cord were calculated. *, p<0.05. (B) Foxp3 expression on CD4+ T cells of CNS in W-sh mice and wild type control. **, p<0.01. (C) Foxp3 expression on CD4+ T cells of the CNS at the different time points in W-sh mice and wild type control. *, p<0.05. Data represent mean values and SD of three separate experiments.
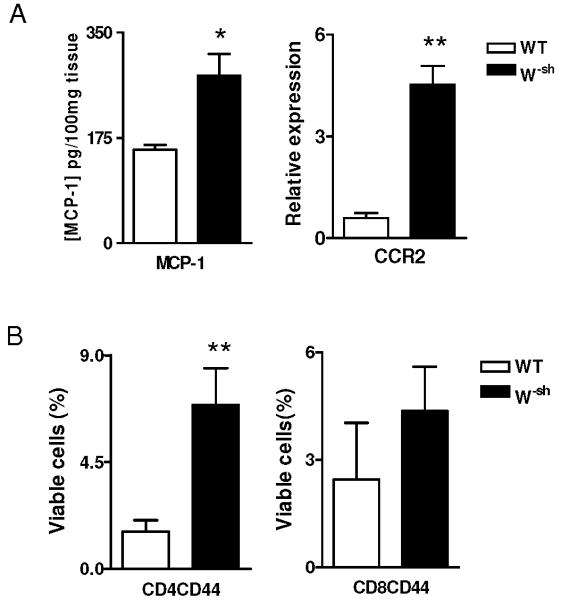
W-sh cells express higher MCP-1/CCR2 and adhesion molecules
To define the mechanism underlying enhanced CNS infiltration in W-sh mice, we determined the production of MCP-1 and the expression of its receptor CCR2, a chemokine/receptor system that is involved in cell infiltration into the CNS (30, 31). CD44 and CD11a are adhesion molecules required for the entry of encephalitogenic T cells into the CNS (32). Although both molecules are expressed at low levels in naive T cells, activated T cells express demonstrably higher levels. Consistent with the increased severity of clinical EAE, we found significantly increased MCP-1 production and CCR2 expression (Fig. 5A) at day 10 p.i., the peak of peripheral T cell responses in wild-type mice. A significantly higher proportion of CD44hiCD4+ T cells was also observed in W-sh mice as compared to wild-type controls, while there was no significant difference in CD44hiCD8+ T cells (Fig. 5B). There was no difference in CD11a expression between the two mouse strains (data not shown).
Fig. 5. W-sh-derived CD4 T cells exhibit increased MCP-1/CCR2 and CD44.
(A) Spinal cord levels of MCP-1. Supernatants of homogenized spinal cords were prepared as described in Materials and Methods and MCP-1 production was assayed by ELISA (n=4 each group; day 10 p.i.). CCR2 expression in spinal cord tissues was determined by real-time RT-PCR. (B) Percentages of CD44hi CD4+ and CD8+ T cells in the spleen at day 10 p.i. were determined by flow cytometry (n=4 each group) *, p<0.05; **, p<0.01. Data represent mean values and SD of three separate experiments.
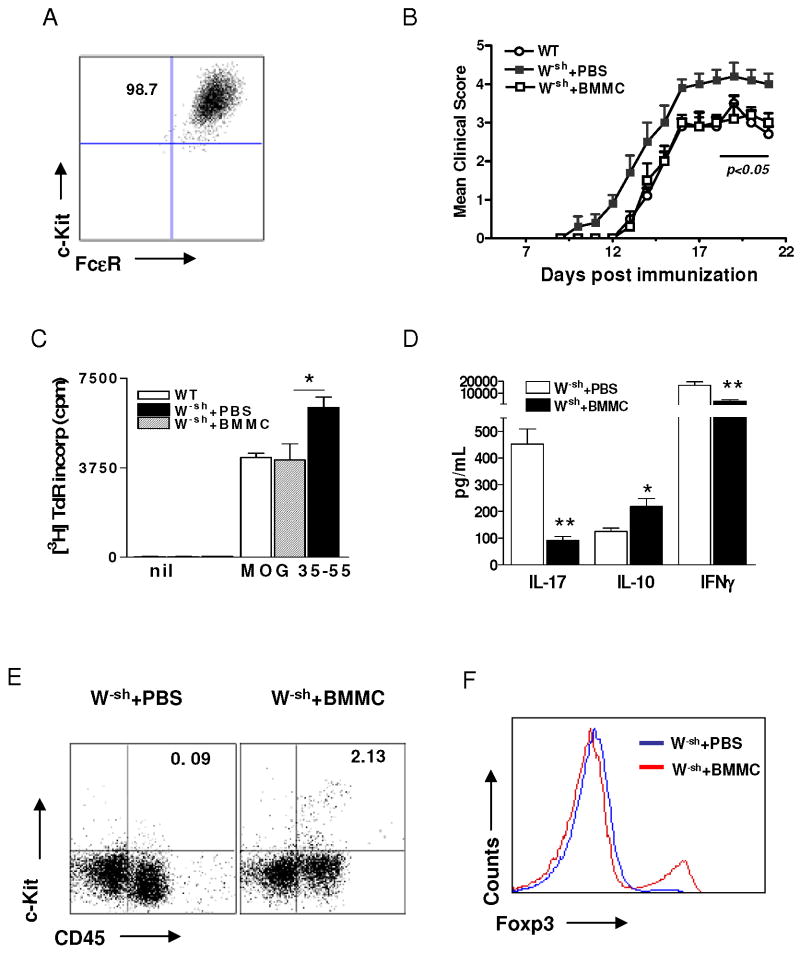
Transfer of bone marrow-derived MCs suppresses clinical EAE in mast cell-deficient mice
To confirm the in vivo regulatory function of MCs, we generated MCs from bone marrow (Fig. 6A) and transferred them into naïve W-sh mice before onset of EAE (Fig. 6B), clinical EAE was downregulated in these reconstituted mice. Proliferative capacity of CD4+ T cells was downregulated in reconstituted W-sh mice (Fig. 6C). Consistent with the clinical scores, splenocytes of mast cell-transferred mice produced significantly lower levels of MOG-induced IL-17 and IFN-γ but higher IL-10 (Fig. 6D), and MC (c-Kit+CD45hi) accumulation in the spinal cord (Fig. 6E), accompanied by increased repopulation of Treg in the CNS (Fig. 6F). These data provide direct evidence that MCs possess immunoregulatory properties.
Fig. 6. Mast cell reconstitution effectively suppresses clinical EAE in mast cell-deficient mice.
MCs were generated from bone marrow of wild-type mice, and the purity was 98.7% by flow cytometry analysis. These cells were then injected i.v. at 5 × 106 to W-sh mice before initiation of EAE. Mice receiving no cells served as control. (A) Flow cytometric analysis of the in vitro differentiated BMMC population. Cells double positive for c-Kit and FcεR1 were considered MCs. (B) Clinical EAE was scored according to a 0∼5 scale as described (n = 5 in each group). (C) At day 10 p.i., mice were sacrificed and splenocytes of mast cell-reconstituted and PBS-treated mice were isolated. Splenocytes were cultured in the presence of MOG35-55 peptide (25 μg/ml) for 3 days. Proliferative responses were measured by [3H] thymidine incorporation. * p<0.05. (D) Splenocytes of mast cell-treated and non-treated W-sh mice were cultured with MOG35-55 peptide for 3 days. Supernatants were assayed for production of IL-17, IL-10, and IFN-γ by ELISA. * p<0.05; ** p<0.01. MC (c-Kit+CD45high) infiltration (E) and Foxp3 expression on CD4+ T cells (F) of CNS in mast cell-reconstituted and PBS-treated mice. Data represent mean values and SD of three separate experiments.
Discussion
In this study, we report that mast cell-deficient W-sh mice develop more severe MOG-induced EAE, which is characterized by earlier onset, more severe paralysis, and more extensive demyelination and inflammatory infiltration than were observed in mast cell-sufficient mice. W-sh mice exhibited elevated proliferative responses to autoantigen, increased production of proinflammatory cytokines/chemokines GM-CSF, IFN-γ, IL-17, MCP-1, increased expression of CD44 on CD4+ T cells and CCR2 on MNCs, and decreased levels of anti-inflammatory cytokines IL-4, IL-5 and IL-10. These observations suggest an immunoregulatory role for MCs in the pathogenesis of CNS inflammatory demyelination.
MCs are classically known for their role in Th2 type immune responses including allergic inflammation, asthma, and anaphylaxis. Heterogeneous MCs can produce an array of both pro- and anti-inflammatory mediators (33), which are known to polarize T-cell differentiation to Th1 (IFN-γ, IL-12), Th2 (IL-4, IL-13), Th17 (IL-6, TGF-β), and Tregs (IL-10, IL-2, TGF-β) (3, 14). Moreover, MCs are required for limiting leucocyte infiltration that associated with skin pathology (34). MCs can mediate the development of T cell tolerance through the release of soluble mediators (34-36). These reports implicate MCs in an ever-widening array of normal and pathological responses, including such diverse processes as allograft tolerance (15), autoimmune arthritis (16, 37, 38), inflammatory bowel disease (17, 39, 40), and VEGF- mediated angiogenesis in tissue repair or driving epithelial cell carcinogenesis (41).
Disease severity of EAE in wild-type and W-sh mice correlated with the degree of CNS inflammation and demyelination. Infiltrating cells were mainly comprised of CD4+ and CD8+ T cells monocytes/macrophages, and neutrophils, which function as effectors and amplifiers of CNS inflammation (42, 43). Of importance is that the total number of DCs in the CNS was increased 2∼3-fold in W-sh mice, in both CD11b+ and CD8α+ DC subsets. Increased numbers of DCs in the CNS may be sufficient to induce more severe inflammation in W-sh mice given that CD8α+ DCs are a more potent DC subset than CD11b+ DCs for initiating Th1-mediated immunopathology (25, 44). Therefore, increased DCs in the CNS, together with significantly increased CD4+ T cells, may be sufficient to induce more severe inflammation in W-sh mice.
MCs have been implicated in the process of DC mobilization and lymph nodes (LN) activation (45-49). Activation of these MCs leads to the production of soluble mediators, such as histamine (50), TNF (51, 52) and IL-1β (49), which promote the migration of peripheral DCs into the LN. However, histamine H4R stimulation of DCs leads to the suppression of IL-12 production but does not alter IL-10 secretion, thereby biasing DCs to have an immunosuppressive character (53). MC-derived TNF-α contributes to maintaining the immunosuppressive environment by increasing antigen presentation in the draining lymph nodes (dLN) in an immunosuppressive manner (54). Our findings, combined with these effects, prove that MCs in EAE may maintain dLN-DC in a tolerizing rather than an immune manner, especially at the onset of disease.
The observation of a greater number of infiltrating cells in the CNS of W-sh mice raises the possibility that MCs may negatively regulate chemokine/chemokine receptor expression. To study this possibility, we determined the expression of MCP-1 in spleen and the CNS and its receptor CCR2, both of which are up-regulated in EAE and MS lesions (55). Although it has been reported that MCP-1 might have an immunoregulatory property (56), the observation that mice lacking MCP-1 exhibited decreased severity of EAE directly supports an inflammatory role of this molecule in EAE (57). MCP-1 present in brain endothelial cells contributes to increased brain endothelial permeability (58). Consistent with the increased severity of clinical EAE, we found significantly increased MCP-1 production and CCR2 expression (Fig. 5) in W-sh mice. Our results are in agreement with the observation that CCR2-/- mice did not develop clinical EAE or CNS histopathology, and showed a significant reduction in T cells and CNS infiltrating monocyte subpopulations (30, 31). As CCR2 is an important determinant of DC migration and localization (59, 60), increased CCR2 would result in more profound DC infiltration in the CNS. Together, these data suggest that up-regulation of CCR2 and MCP-1 in W-sh mice may promote up-regulation of DC infiltration in the CNS, thus enhancing autoimmune inflammatory infiltration.
MCs are present in small numbers within secondary lymphoid organs, and their number increases in lymph nodes in response to immune challenge (4, 13). It has been recently observed that the number of MCs increases in bone marrow biopsy specimens in parallel with the increase of Tregs (61). An increased mast cell population in Treg-rich lymphoid infiltrates suggests that Tregs recruit and activate MCs in situ (15, 61). It has been shown that transfer of Tregs enhanced survival, resulting in a marked increase in the number of MCs in the peritoneal cavity (62). Fujita T et al. demonstrated that IFN-α treatment of FcεR1-activated human MCs caused a shift from TNF to an enhanced production of IL-10 and TGF-β by T cells, with a concomitant decrease in OX40L expression (63). IL-10 and TGF-β affected Treg cell differentiation and effector function (64), and induced alloreactive CD4+CD25- T cells to acquire regulatory cell function (65). At the same time, the decrease in OX40L expression may stabilize Treg lineage commitment and their suppressive activity (11). Also, another study has reported that MC deficiency can directly down-regulate expansion of Treg cells (10). In this study we verified that the T cell compartment of naive W-sh mice was intact and that there were no intrinsic differences in T cell development in the thymus or in peripheral T cell populations compared with their wild-type counterparts (data not shown). It was only after MOG35-55 immunization that the enhancement in T cell responses and decreased Foxp3 expression in infiltrating CD4+ T cells in W-sh mice were evident. These data confirm a role for MCs in the generation of antigen-specific T cell responses in vivo and are consistent with the report that MCs play a regulatory role in inflammation and autoimmune diseases (15-17). Further, we found that the proportion of CD44hi cells was significantly higher on CD4+ T cells in splenocytes of W-sh mice at early time points post immunization. Thus, the characterization of peripheral immune-cell cytokine response to MOG35-55 demonstrated that W-sh mice generate enhanced effector T cell responses, with simultaneous reduction in antagonizing anti-inflammatory responses. These results, together with observations from others (15, 37), suggest that MCs play an important role in regulating T cell functions.
IL-4, IL-13, IL-5 and IL-6 have been localized to MCs at both the protein and mRNA levels. MCs have been named as the source of the large majority of these cytokines (66-68). It has been reported that heterogeneity in MCs is based on cytokine expression (69). MCs release Th2 cytokines independent of T cell help (70). However, although T cells are known to be an important source of cytokines, they require activation and, in the case of Th2 cells, priming with IL-4 before they can release them (71, 72). Moreover, Anderson et al. showed that MCs can skew the development of a Th2-like immune response before Ag-mediated activation (73). Thus, the early onset and severe disease in W-sh mice may explain, at least in part, the decreased Th2 immune response in these mice.
The difference between our results and those of Secor et al. (19) may be due to differences in the mast cell-deficient mouse strains used. In addition to mast cell deficiency, the reduced c-Kit function in W/W-v mice results in many other phenotypic abnormalities. These include macrocytic anemia, impaired melanogenesis, a virtual lack of interstitial cells of Cajal, and a decline in the number of intestinal TCRγδ intraepithelial lymphocytes with age. W/W-v mice also develop a high incidence of spontaneous inflammation, such as dermatitis (74). These abnormalities may result in unexpected immune deficits and skew conclusions, including these in EAE. Recently, W-sh mice have been used as a model for studies of mast cell function in vivo (75). Phenotypic characterization of W-sh mice indicates that they have an increased number of circulating neutrophils and an enlarged spleen (76, 77). In the spleen W-sh animals have abundant neutrophils, an increased number of F4/80 positive cells (macrophages) and megakaryocytes, while showing a normal number of DC (76). In the bone marrow cell population W-sh animals also show an increased number of both CD45 + and F4/80 positive cells. Therefore, the data presented by Secor et al. and those of the present paper cannot be directly compared as they relate to the role played by MC. Indeed, it has been recently found that W/Wv mice are resistant to anti-collagen/LPS-induced arthritis, while W-sh mice are susceptible, suggesting that other host differences determine the extent of MC involvement (37).
Given that both neutrophils and macrophages play a key role in the pathogenesis of EAE (78), haematopoietic changes between MC-deficient strains could make a difference. Depletion of MC in WBB6 could not influence neutrophil or monocyte recruitment, while in W-sh, MCs could allow for a stronger reaction of Teff, which may be phagocyte-mediated. The absence of MCs in W-sh animals may, for example, allow a more active neutrophilic (or macrophagic) reaction.
In summary, we found that MCs have a suppressive role in EAE. Mechanisms underlying more severe CNS inflammation include increased generation of Th17 cells and production of proinflammatory cytokines, elevated MCP-1/CCR2 expression, decreased production of anti-inflammatory cytokines IL-4, IL-5, IL-10 and IL-27, and decreased numbers of CD4+Foxp3+ Tregs. The demonstration that the absence of MCs can enhance the severity of EAE should encourage further study of these cells in experimental and human autoimmunity.
Acknowledgments
We thank Katherine Regan for editorial assistance.
Grant Support: This work was funded by NIH and by a postdoctoral fellowship to DCF from the National Multiple Sclerosis Society.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- MOG35–55
peptide 35-55 of myelin-oligodendrocyte glycoprotein
- MNCs
mononuclear cells
Footnotes
Disclosures: The authors have nothing to disclose
References
- 1.Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak AM. New aspects of mast cell biology. Int Arch Allergy Immunol. 1997;114:1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 5.Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daeron M. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465–6475. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 8.Frandji P, Oskeritzian C, Cacaraci F, Lapeyre J, Peronet R, David B, Guillet JG, Mecheri S. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J Immunol. 1993;151:6318–6328. [PubMed] [Google Scholar]
- 9.Frandji P, Tkaczyk C, Oskeritzian C, David B, Desaymard C, Mecheri S. Exogenous and endogenous antigens are differentially presented by mast cells to CD4+ T lymphocytes. Eur J Immunol. 1996;26:2517–2528. doi: 10.1002/eji.1830261036. [DOI] [PubMed] [Google Scholar]
- 10.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Caton AJ, Koretzky GA. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 12.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsythe P, Befus AD. CCR3: a key to mast cell phenotypic and functional diversity? Am J Respir Cell Mol Biol. 2003;28:405–409. doi: 10.1165/rcmb.F265. [DOI] [PubMed] [Google Scholar]
- 14.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 15.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel TA, Sampaio AL, D'Acquisto F, Perretti M, Oliani SM. An essential role for mast cells as modulators of neutrophils influx in collagen-induced arthritis in the mouse. Lab Invest. doi: 10.1038/labinvest.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietsch GN, Hinrichs DJ. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- 21.Brenner T, Soffer D, Shalit M, Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J Neurol Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 22.Levi-Schaffer F, Riesel N, Soffer D, Abramsky O, Brenner T. Mast cell activity in experimental allergic encephalomyelitis. Mol Chem Neuropathol. 1991;15:173–184. doi: 10.1007/BF03159954. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2009;182:5507–5514. doi: 10.4049/jimmunol.0801485. [DOI] [PubMed] [Google Scholar]
- 24.Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, Whitacre CC. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest. 2000;106:1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Amarnath S, Chen W. Requirement of CD28 signaling in homeostasis/survival of TGF-beta converted CD4+CD25+ Tregs from thymic CD4+CD25- single positive T cells. Transplantation. 2006;82:953–964. doi: 10.1097/01.tp.0000232330.46688.37. [DOI] [PubMed] [Google Scholar]
- 28.Song F, Wardrop RM, Gienapp IE, Stuckman SS, Meyer AL, Shawler T, Whitacre CC. The Peyer's patch is a critical immunoregulatory site for mucosal tolerance in experimental autoimmune encephalomylelitis (EAE) J Autoimmun. 2008;30:230–237. doi: 10.1016/j.jaut.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JP, Morris-Downes M, Brennan FR, Wallace GJ, Amor S. A role for alpha4-integrin in the pathology following Semliki Forest virus infection. J Neuroimmunol. 2000;106:60–68. doi: 10.1016/s0165-5728(99)00235-0. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Ishizuka T, Okayama Y. Human mast cells and basophils as sources of cytokines. Clin Exp Allergy 30. 2000:1205–1212. doi: 10.1046/j.1365-2222.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 34.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 35.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 37.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 39.Araki Y, Andoh A, Fujiyama Y, Bamba T. Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin Exp Immunol. 2000;119:264–269. doi: 10.1046/j.1365-2249.2000.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rijnierse A, Koster AS, Nijkamp FP, Kraneveld AD. Critical role for mast cells in the pathogenesis of 2,4-dinitrobenzene-induced murine colonic hypersensitivity reaction. J Immunol. 2006;176:4375–4384. doi: 10.4049/jimmunol.176.7.4375. [DOI] [PubMed] [Google Scholar]
- 41.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 43.Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, Ruddle NH. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2000;164:419–426. doi: 10.4049/jimmunol.164.1.419. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–1762. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 46.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 47.Demeure CE, Brahimi K, Hacini F, Marchand F, Peronet R, Huerre M, St-Mezard P, Nicolas JF, Brey P, Delespesse G, Mecheri S. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol. 2005;174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 48.Wasiuk A, de Vries VC, Hartmann K, Roers A, Noelle RJ. Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, Bopp T, Taube C, Schild H, Schmitt E, Stassen M. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood. 2007;110:946–953. doi: 10.1182/blood-2006-07-036889. [DOI] [PubMed] [Google Scholar]
- 50.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol. 2004;173:5275–5282. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 51.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 52.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittmann M, Werfel T. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 54.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 55.Quinones MP, Kalkonde Y, Estrada CA, Jimenez F, Ramirez R, Mahimainathan L, Mummidi S, Choudhury GG, Martinez H, Adams L, Mack M, Reddick RL, Maffi S, Haralambous S, Probert L, Ahuja SK, Ahuja SS. Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB and Akt. Mol Cell Neurosci. 2008;37:96–109. doi: 10.1016/j.mcn.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elhofy A, Wang J, Tani M, Fife BT, Kennedy KJ, Bennett J, Huang D, Ransohoff RM, Karpus WJ. Transgenic expression of CCL2 in the central nervous system prevents experimental autoimmune encephalomyelitis. J Leukoc Biol. 2005;77:229–237. doi: 10.1189/jlb.0804465. [DOI] [PubMed] [Google Scholar]
- 57.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ransohoff RM. Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leukoc Biol. 1997;62:645–652. doi: 10.1002/jlb.62.5.645. [DOI] [PubMed] [Google Scholar]
- 59.Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J Exp Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurent C, de Paiva GR, Ysebaert L, Laurent G, March M, Delsol G, Brousset P. Characterization of bone marrow lymphoid infiltrates after immunochemotherapy for follicular lymphoma. Am J Clin Pathol. 2007;128:974–980. doi: 10.1309/LREBX069UXDYMBXV. [DOI] [PubMed] [Google Scholar]
- 62.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 63.Fujita T, Kambe N, Uchiyama T, Hori T. Type I interferons attenuate T cell activating functions of human mast cells by decreasing TNF-alpha production and OX40 ligand expression while increasing IL-10 production. J Clin Immunol. 2006;26:512–518. doi: 10.1007/s10875-006-9043-1. [DOI] [PubMed] [Google Scholar]
- 64.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–276. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 65.Chen ZM, O'Shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, Roncarolo MG, Blazar BR. IL-10 and TGF-beta induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 66.Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, Howarth PH. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993;151:3853–3865. [PubMed] [Google Scholar]
- 67.Bradding P, Mediwake R, Feather IH, Madden J, Church MK, Holgate ST, Howarth PH. TNF alpha is localized to nasal mucosal mast cells and is released in acute allergic rhinitis. Clin Exp Allergy. 1995;25:406–415. doi: 10.1111/j.1365-2222.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 68.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bradding P, Okayama Y, Howarth PH, Church MK, Holgate ST. Heterogeneity of human mast cells based on cytokine content. J Immunol. 1995;155:297–307. [PubMed] [Google Scholar]
- 70.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 72.Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, Bews JP, Hunt TC, Okayama Y, Heusser CH, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson DF, Zhang S, Bradding P, McGill JI, Holgate ST, Roche WR. The relative contribution of mast cell subsets to conjunctival TH2-like cytokines. Invest Ophthalmol Vis Sci. 2001;42:995–1001. [PubMed] [Google Scholar]
- 74.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kihara Y, Yokomizo T, Kunita A, Morishita Y, Fukayama M, Ishii S, Shimizu T. The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 394:673–678. doi: 10.1016/j.bbrc.2010.03.049. [DOI] [PubMed] [Google Scholar]