Fig. 1.
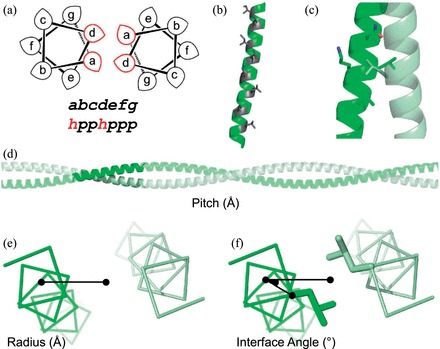
Sequence and structural features of the α-helical coiled coil. (a) Helical wheel representing a coiled-coil dimer. Hydrophobic residues are typically located at the a and d positions, with polar residues at the b, c, e, f and g positions. (b) A single helix from a coiled coil, highlighting the stripe of a/d residues (grey) that forms the assembly interface. (c) KIH packing, where the knob residue (usually a or d) from one helix (grey) projects into a hole created by four residues (black) on its partner (e.g. d’g’a’d’ or a’d’e’a’). (d) The pitch parameter describes the distance (Å) for a component helix to screw 360° around the super-helical axis. (e) The radius of assembly is measured from the super-helical axis to the centre of a component helix. (f) Interface angle, or ϕ1, is measured as the angle between the vector from the super-helical axis to the helical centre and the vector from the helical centre to the Cα carbon of an a-position residue
