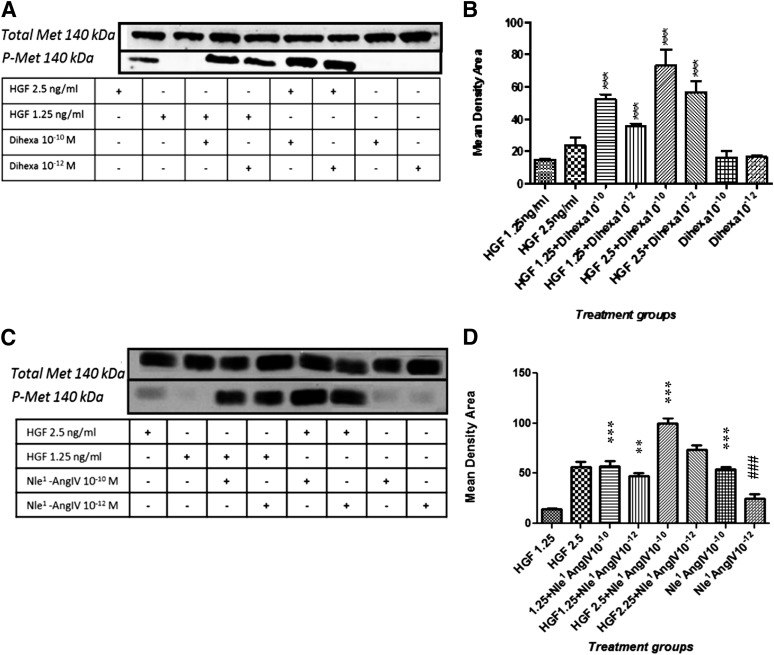
Fig. 2.
The HGF mimetics dihexa and Nle1-AngIV allosterically regulate HGF, resulting in c-Met activation. The effect of dihexa and Nle1-AngIV on HGF-dependent c-Met activation was assessed in HEK-293T cells and analyzed for phosphorylated (activated) and total c-Met by immunoblotting. (A) An SDS-PAGE illustrates the impact of dihexa at 10–10 M and 10–12 M on HGF-dependent c-Met phosphorylation. (B) Dihexa at 10–10 M augmented the effect of HGF at 1.25 ng/ml (P < 0.001) and 2.5 ng/ml (P < 0.001). Similarly, dihexa at 10–12 M augmented HGF’s activity at 1.25 ng/ml (P < 0.001) and 2.5 ng/ml (P < 0.001). Neither dihexa at 10–10 M nor at 10–12 M stimulated c-Met phosphorylation beyond background. No significant change in total c-Met was observed in any experimental group. N = 4; mean ± S.E.M. (C) An SDS-PAGE illustrates the impact of Nle1-AngIV at 10–10 M and 10–12 M on HGF-dependent c-Met phosphorylation. (D) Nle1-AngIV at 10–10 M (***P < 0.001) and 10–12 M (**P < 0.01) augmented the effect of HGF at 1.25 ng/ml, and Nle1-AngIV at 10–10 M and 10–12 M augmented HGF’s activity at 2.5 ng/ml (***P < 0.001). Nle1-AngIV alone at 10–10 M stimulated c-Met phosphorylation beyond background (P < 0.05) while Nle1-AngIV at 10−12 M was not different from background (###P > 0.05). No significant change in total c-Met was observed in any experimental group. N = 4; mean ± SEM. Together these data demonstrate the capacity of HGF mimetics to shift the dose-response curve of HGF’s activation of c-Met to the left.