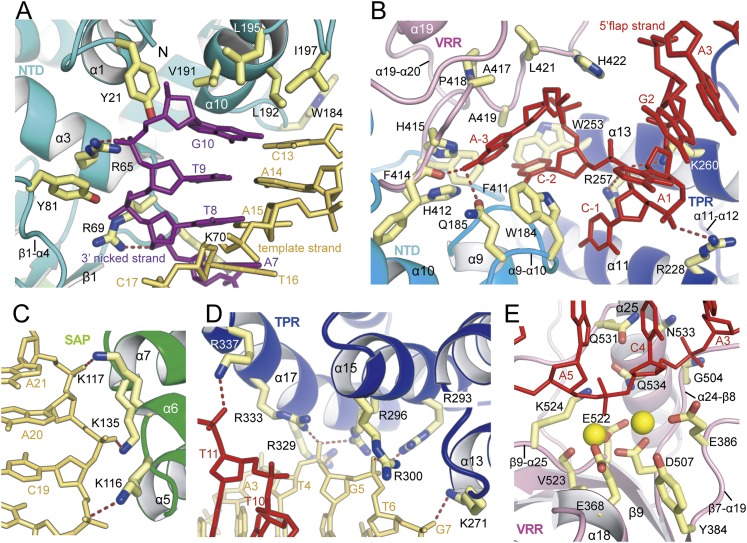
Figure 3.
Close-up view of the interactions between the FAN1 and DNA substrate. (A) Close-up view of the prenick segment binding to the NTD. Four hydrophobic residues at the α10 wedge face the terminal bases G10:C13, and tyrosine residues and basic residues make contact with the phosphate oxygen atoms of the 3′ nicked and template strands. (B) Recognition of the 5′ flap part by the pocket composed of TPR domain (purple), VRR nuc domain (pink), and NTD (cyan). Recognition of the 5′ flap by FAN1 is augmented via binding of the phosphate of C-1 by the α13 helix (R257 and K260) and of the phosphate of A1 by the α11 helix (R228) See also Figure 2I and Supplemental Figure 3. (C) Three bases (K116, K135, and K117) from the SAP domain recognize the phosphate oxygen atoms adjacent the 5′ end of the template strand. (D) Recognition of the post-nick segment by the four inner layer helices from the TPR domain. Basic residues from the α15 and α17 helices bind the phosphate groups near the 3′ end of the template strand, whereas the α11 and α13 helices participate in binding to the 5′ flap region. K271 (α13) binds the phosphate oxygen atoms of G7. R293, R296, and R300 (α15) interact with the phosphate oxygen atoms of T6, and R296, R329, and R333 (α17) bind the phosphate oxygen atoms of G5 of the template strand. (E) The incised phosphodiesters bound to the active site at the VRR nuc domain. The 4.0 Å metal-bound PaFAN1–DNA structure is shown. Two metal ions are shown as yellow spheres. The A5 is 5 nt downstream from the junction between the prenick and post-nick DNA. For an overall scheme of the FAN1–DNA interaction, see Figure 2I.