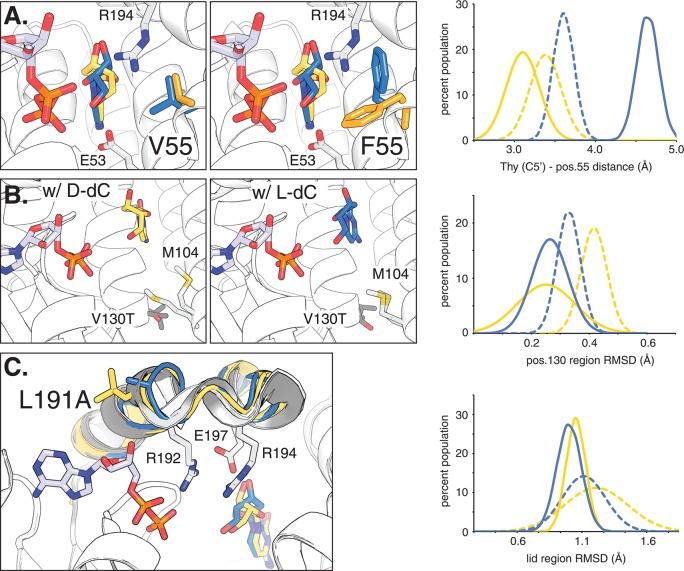
Figure 4.
Active site models based on molecular dynamics simulation of functional amino acid substitutions in dCK variants. For reference, ADP is bound in the phosphoryl donor site. The overlaid structures of d- and l-dC in the phosphoryl acceptor binding pocket are highlighted in yellow and blue, respectively. (A) Comparison of parental ssTK3 (V55) and variant B8 (F55) with their dominant side chain orientations at position 55 highlighted in yellow (for d-isomer) and blue (for l-isomer). Neighboring residues E53 and R194 are marked. The graph shows the overall distribution of preferred side chain conformations as a measure of the distance between the substrate C5′ position and nearest side chain atom for ssTK3 (dotted lines) and B8 (solid lines). (B) Effect of V130T substitution on M104 side chain formation in the presence of d-dC and l-dC, respectively. The graph shows the lower conformational flexibility of residues near T130 (variant B7) versus V130 (B0) via the region’s root square mean deviation (RMSD). (C) Impact of L191A substitution on the enzyme’s lid region (highlighted in gray) and its three catalytic residues (R192, R194, and E197). The reduced conformational flexibility of variant B6 (A191) compared to parent ssTK3 (L191) is reflected in the lower, more narrow RMSD distribution.