Figure 2.
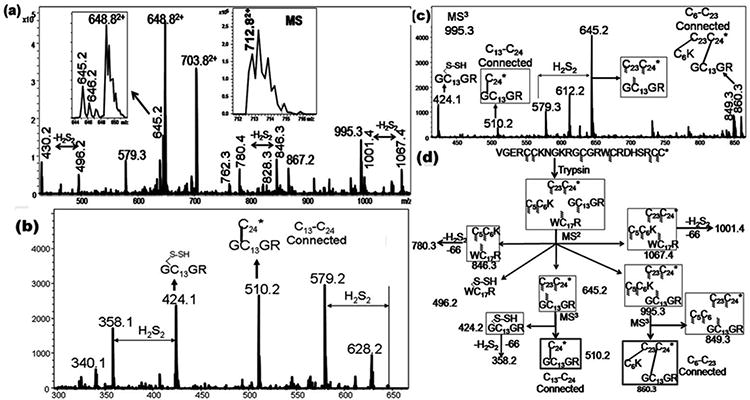
(A) CID MS2 spectra of the doubly charged species (712.8, (M+2H)2+) of the tryptic peptide of μ‐BuIIIB. Inset shows the MS of the precursor ion. (B) CID MS3 spectra of 645.5. (C) CID MS3 spectra of 995.5. (D) Assignments of the key MSn fragment ions of tryptic Bu-IIIB.The m/z values of each of the ions are indicated against the respective structures and they correspond to the singly charged values, unless otherwise specified. For every structure, Cys residues with indeterminate connectivity are indicated with the wavy lines. Subsequently, the structures from which a particular Cys connectivity is evident, the connected Cys residues are joined through a line. In this case, while the C13‐C24 connectivity is established by the MS3 ion 510.2, the ion 860.3 confirms the C6‐C23 connectivity.
