Abstract
Squamous cell carcinoma of the palm is a very infrequent malignancy. Its unusual presentation can produce a delay in the final diagnosis with serious consequences as far as morbidity and mortality are concerned. This article summarises the case of a patient who was referred to our department presenting a squamous cell carcinoma on his left palm and a clinically positive axillar lymphadenopathy. He had previously been wrongly diagnosed on several occasions.
Background
Cutaneous-origin malignant neoplasms can appear in any layer of the skin. One per cent to 2% of primary tumours of the hand are malignant. Among these, cutaneous squamous cell carcinoma (SCC) is the most common, accounting for 58–90% of all hand tumours.1 2 Approximately 15% of all SCC tumours appear in the hand.3 4 Interestingly, the development of SCCs of the palm is extremely rare, especially when their frequency is compared with that of the rest of the hand.5 This fact could lead to misdiagnosis or delayed diagnosis.
Case presentation
A 63-year-old white male farmer was referred to us for evaluation and management of an ulcerated lesion on the palm of his non-dominant left hand.
Eight years earlier, the patient had sustained an accidental puncture in the same location with a thorn while handling wild gorse bushes. The patient noted that the wound never healed completely and he was referred to multiple inflammatory-suppurative processes in the next 7 years. Several health providers of different specialties and institutions treated the lesion conservatively as a pyogenic granuloma in the past 2 years. Therefore, three surgical explorations, cleansings and debridements were carried out, but despite intermittent periods of improvement, the wound remained ulcerated and colonised.
On physical examination in our department the patient presented a deeply ulcerated, painful wound of 5×4 cm affecting the ulnar half of his left palm, with hypergranulated foul-smelling sinus and surrounding fluctuant tissues (figure 1). One month prior to referral, the patient noted the development of a fast-growing axillary mass (figure 2).
Figure 1.
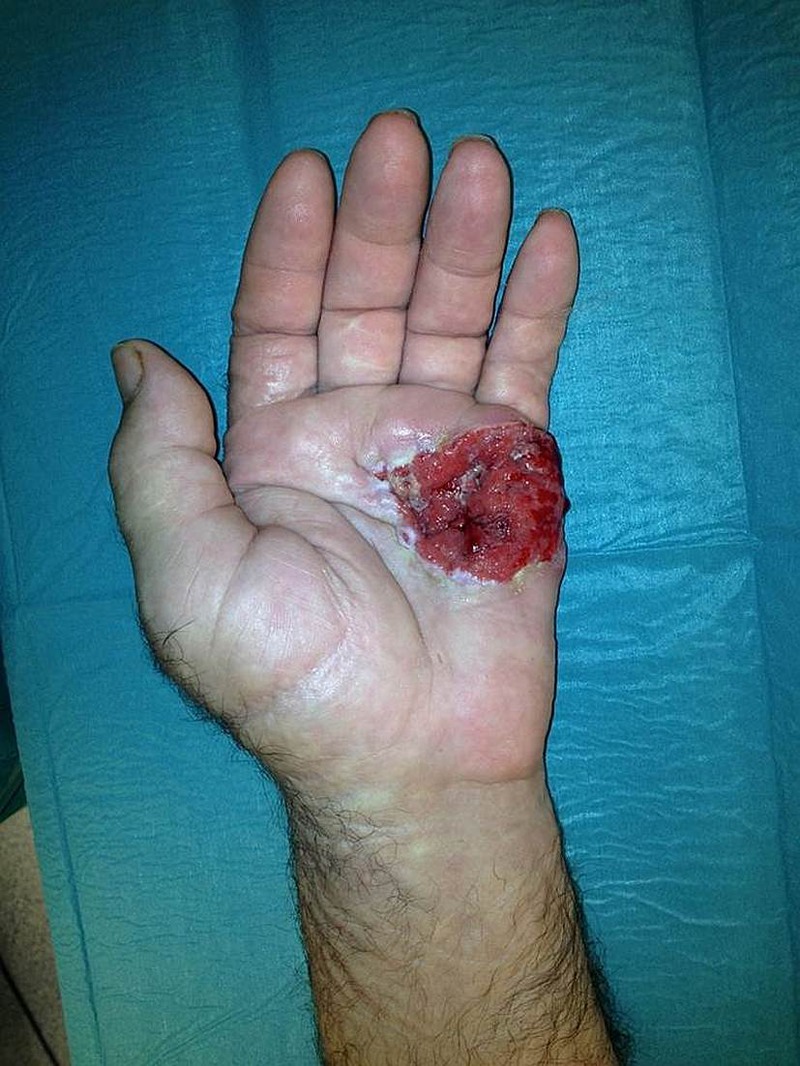
View of the palmar tumour at physical examination 13 months after initial presentation.
Figure 2.
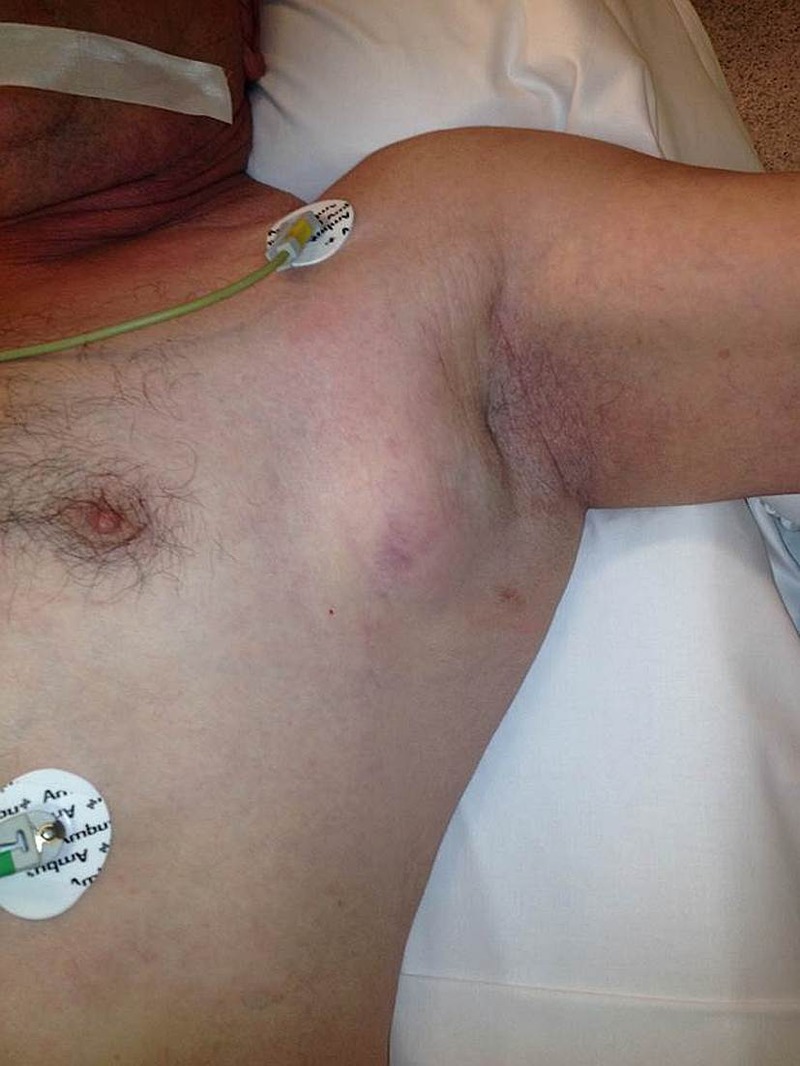
Painless, fixed axillary subcutaneous mass just before surgical dissection.
Investigations
Histopathological study of the hand ulcer and axillary nodes was indicated in order to confirm the previous diagnosis of pyogenic granuloma. The histological examination of incisional biopsies of the ulcer and fine needle aspiration biopsy of the nodes was consistent with poorly differentiated cutaneous SCC.
Once the histology confirmed the neoplastic process, MRI of the hand and CT scan were realised. MRI revealed the mass deepening from the ulcer to infiltrate the subcutaneous fat, flexor tendons, neurovascular bundles and intrinsic muscles on the volar aspect of third, fourth and fifth rays. The CT scan showed a conglomerate of axillary masses strongly suggestive of necrotic adenopathies with no evidence of distant metastases.
Treatment
Axillary node dissection was performed and revealed a moderately differentiated SCC of metastasis origin in 24 of 41 lymphadenopathies examined (figure 3). The patient chose a limb salvage procedure and a triple-ray amputation of the third, fourth and fifth fingers was carried out including a 1 cm skin margin. Although the mass extended through the soft tissue around the third metacarpal, it was possible to preserve the common intermetacarpal nerve of the second space. The proximal margin of the resection was at the level of the carpometacarpal joint. The defect was covered by means of a previously designed dorsal fasciocutaneous flap drawing on the non-affected dorsal tissues of the ulnar amputation sample. The histology of the surgical sample reported a moderately differentiated SCC (figures 4 and 5), along with an extensive inflammatory infiltrate and clear disease-free margins.
Figure 3.
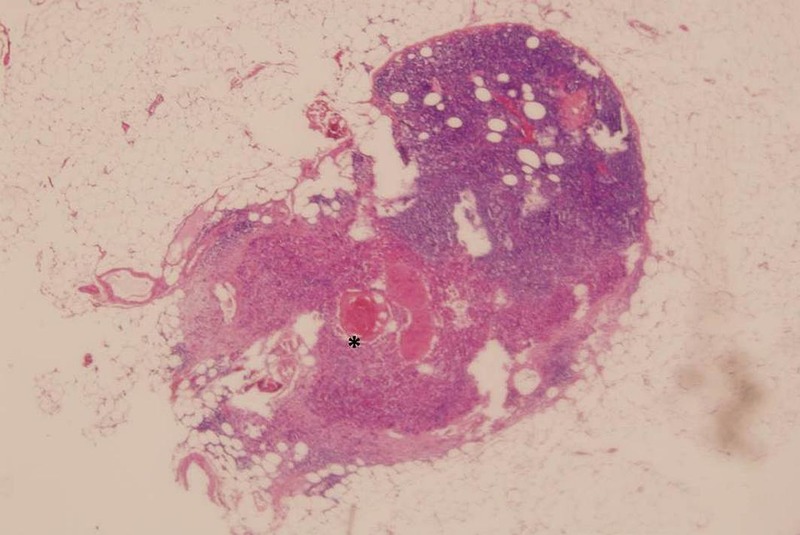
Axillary lymph node squamous cell carcinoma metastasis (*), H&E (×20).
Figure 4.
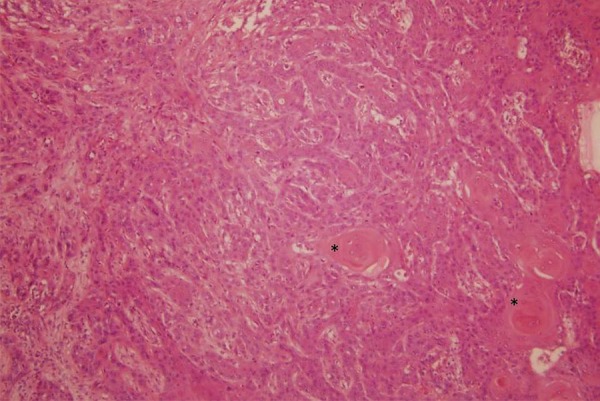
Tumoural proliferation in the dermis with epithelial-like cells and focal keratinisation (*), H&E (×100).
Figure 5.
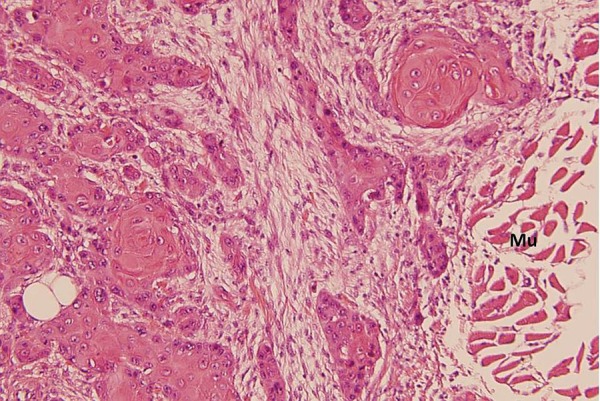
Peripheral invasion of the muscular tissue (Mu), H&E (×200).
Outcome and follow-up
A positron emission tomography scan was performed to ensure the absence of distant metastases and the patient was referred to the medical oncology department in order to consider further non-surgical treatment.
A month and a half after surgery the patient was able to achieve a full range of movement and an acceptable precision pinch between the first and second finger for daily activities, although power grasp was lost (figure 6).
Figure 6.
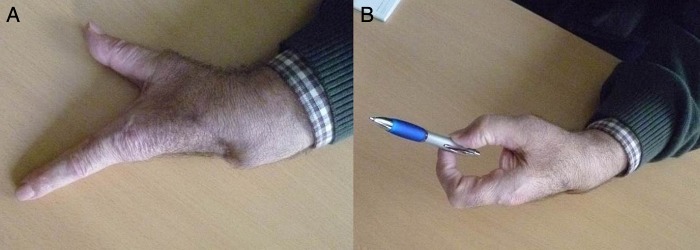
Functional outcome 48 days after surgery. Full extension of the first and second ray is easily achieved (A) with an acceptable precision pinch (B).
Discussion
Skin SCC of the volar side of the hand is an infrequent malignancy. In this particular case it was important to pay attention during the long course of recurrent and non-completely healing ulcers that the patient referred at the side of an old accidental wound. It has been proposed that inflammatory processes that occur repeatedly are due to infections or burn scarring.6–8 Treves and Pack attempted to explain the ulceration and malignisation of burn scars as due to the effects that the toxins of the damaged tissue had on the scar itself by way of autolysis or heterolysis.9 Castillo and Goldsmith,10 followed by Bostwick et al,11 developed hypotheses relating to malignisation due to the absence of a normal anatomy of the lymphatic system in the scar, which would allow the tumour to develop without the influence of the defence mechanisms of the immune system.
Wide local excision (WLE) is indicated to treat either low-risk or high-risk SCC. For standard WLE, the recommended excision margin for high-risk SCC is at least 6 mm, and for low-risk SCC, a margin of at least 4 mm. Although the neoplasm is limited to the skin, the excision should be taken down to the subcutaneous fat12 or to a depth one layer below the tumour along with any adjacent area of induration.13 The literature includes diverse studies reviewing the treatment of SCC of the head and neck, but there are few studies focusing on the outcomes of surgical excision of SCC of the hand. Some surgeons are led to consider amputation as a treatment for aggressive tumours due to cases reported in the literature. However, the use of amputation as a treatment for such tumours is currently controversial in terms of survival. Rayner14 showed a 21% rate of death from metastasis with amputation versus a 7% rate with radiation therapy alone in a retrospective review of 273 patients with SCC of the hand.
In general, there is no indication to perform routine prophylactic lymph node dissection in SCC of the hand.15 The practice of sentinel lymph node biopsy (SLNB) is well established in staging and treating melanoma and breast cancer. However, for the treatment of SCC of the hand its indications are less clear.13 SLNB may be of benefit for patients with high-risk tumours, as suggested in various reports, but there is no decisive evidence on higher survival rates brought about by the standard use of SLNB for patients with cutaneous SCC of the hand.15
Most patients with SCC have a good prognosis. Low-risk tumours are cured by WLE and are less prone to developing nodal metastasis. However, high-risk tumours augur a less favourable prognosis,2 16 with a risk of metastasis as high as 48%. Furthermore, patients with nodal metastasis have shown 3-year and 5-year survival rates of 69% and 48%, respectively.16
Learning points.
Squamous cell carcinomas of the palm are extremely infrequent and could be misdiagnosed due to confusion with more common pathologies.
Suspicion of malignancy must be taken into account in chronic non-healing ulcers. Infectious process can be synchronous and be a cause of misdiagnosis.
Incisional or excisional biopsies must be carried out in order to obtain an early diagnosis in an attempt to avoid aggressive surgery in the future, including amputation, and to reduce morbidity and mortality.
It has not been proved that limb sparing surgery with wide local excision in squamous cell carcinomas of the hand has an influence on the rate of relapses and mortality.
Acknowledgments
The authors would like to thank Dr Elena Pintos MartÃnez MD for her time and valuable support with histopathological pictures.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Askari M, Kakar S, Moran SL. Squamous cell carcinoma of the hand: a 20-year review. J Hand Surg Am 2013;38:2124–33 [DOI] [PubMed] [Google Scholar]
- 2.Martin DE, English JC III, Goitz RJ. Squamous cell carcinoma of the hand. J Hand Surg Am 2011;36:1377–81 [DOI] [PubMed] [Google Scholar]
- 3.Ağir H, Adams BM, Mackinnon CA. Squamous cell carcinoma of the palm: a case report. Acta Orthop Traumatol Turc 2007;41:321–5 [PubMed] [Google Scholar]
- 4.Tamurian RM, Gutow AP. Amputations of the hand and upper extremity in the management of malignant tumors. Hand Clin 2004;20:213–20 [DOI] [PubMed] [Google Scholar]
- 5.Terkonda SP, Perdikis G. Non-melanotic skin tumors of the upper extremity. Hand Clin 2004;20:293–301 [DOI] [PubMed] [Google Scholar]
- 6.Da Costa JC. Carcinomatous changes in an area of chronic ulceration, or Marjolin's ulcer. Ann Surg 1903;37:496–502 [PMC free article] [PubMed] [Google Scholar]
- 7.Hill BB, Sloan DA, Lee EY, et al. Marjolin's ulcer of the foot caused by non-burn trauma. South Med J 1996;89:707–10 [DOI] [PubMed] [Google Scholar]
- 8.Ozek C, Cankayali R, Bilkay U, et al. Marjolin's ulcers arising in burn scars. J Burn Care Rehabil 2001;22:384–9 [DOI] [PubMed] [Google Scholar]
- 9.Treves N, Pack GT. Development of cancer in burn scars. Surg Gynecol Obstet 1930;51:749–82 [Google Scholar]
- 10.Castillo JL, Goldsmith HS. Burns scar malignancy in a possible depressed immunologic setting. Surg Forum 1968;19:511–13 [PubMed] [Google Scholar]
- 11.Bostwick J, Pendergrast WJ, Vasconez LO. Marjolin's ulcer: an immunologically privileged tumor? Plast Reconstr Surg 1976;57:66–9 [PubMed] [Google Scholar]
- 12.Ilyas EN, Leinberry CF, Ilyas AM. Skin cancers of the hand and upper extremity. J Hand Surg Am 2012;37:171–8 [DOI] [PubMed] [Google Scholar]
- 13.English C, Hammert WC. Cutaneous malignancies of the upper extremity. J Hand Surg Am 2012;37:367–77 [DOI] [PubMed] [Google Scholar]
- 14.Rayner CR. The results of treatment of two hundred and seventy-three carcinomas of the hand. Hand 1981;13:183–6 [DOI] [PubMed] [Google Scholar]
- 15.Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. Malignant. Dermatol Surg 2007;33:771–85 [DOI] [PubMed] [Google Scholar]
- 16.Johnston EA, Namm JP, Reeves ME. Major extremity amputation for nodal metastasis from squamous cell carcinoma. J Surg Oncol 2006;93:76–8 [DOI] [PubMed] [Google Scholar]


