Abstract
Multiple myeloma (MM) is the second most common haematological malignancy in the UK. We present a case series of three patients with light chain only myeloma who had normal serum protein electrophoretic studies at screening and were diagnosed using serum and urine free light chain assessment. This series reiterates the importance of thorough and robust screening for MM in patients presenting with renal disease. We review the up to date literature and we highlight the need to screen patients for MM with a combination of serum electrophoresis/immunofixation and either urinary or serum free light chain measurement and to maintain a high index of suspicion regardless of the presence or absence of proteinuria. We also discuss the emerging role of the serum free light chain assay.
Background
Multiple myeloma (MM) is the second most common haematological malignancy with between 4500 and 4800 new cases diagnosed in the UK each year.1 Diagnosis of MM is based on the presence of excess plasma cells in the bone marrow, a monoclonal paraprotein in the serum or urine and related organ or tissue impairment such as hypercalcaemia, renal insufficiency, anaemia or bone lesions.2 In approximately 20% of cases, the paraprotein consists of free light chains only and may not be associated with a paraprotein spike on serum electrophoretic studies.3 Renal failure has been shown to occur in over half of patients with light chain disease.4 Circulating nephrotoxic free light chains have pathological effects in the glomerular and tubulointerstitial compartments of the kidney. We present three cases of patients with light chain only myeloma who have presented within the last year with normal serum protein electrophoretic studies at screening and who were diagnosed using serum and urine free light chain assessment. These cases reiterate the need for exclusion of free light chain only disease when screening for plasma cell dyscrasias. We also review up to date evidence on the need for testing of urine and serum free light chain (sFLC) assays with a view to rationalising screening for MM.
Case presentation
Case 1
A 53-year-old man was seen in the nephrology outpatient clinic with deteriorating renal function. He had been short of breath for approximately 6 months, but prior to that his exercise tolerance had been unlimited. His significant medical history consisted of asthma, hypercholesterolaemia and pericarditis with moderate non-steriodal anti-inflammatory drug (NSAID) use for 4 months. His blood pressure was 140/70 mm Hg. Examination of the cardiovascular, respiratory and abdominal systems were unremarkable.
Case 2
A 66-year-old man was referred to the nephrology outpatient clinic with declining renal function over 2 months. He had noticed decreasing appetite and lethargy but reported no other symptoms. His background included alcoholic liver disease with cirrhosis (now abstinent), type 2 diabetes mellitus, hypertension and a previous stoke. He had previously been a smoker. His blood pressure was 165/80 mm Hg. Palmar erythema was noted on examination with upper limb bruising and tattoos. He had telangiectasia on his chest. There was no asterixis. He had peripheral oedema but the jugular venous pressure was not raised. Abdominal examination revealed some ascites. Examination of the cardiovascular and respiratory systems was unremarkable.
Case 3
A 67-year-old man was transferred to the nephrology unit after he presented to his local hospital with a serum creatinine of 608 mmol/L. Prior to admission to the hospital, he had been feeling unwell with lethargy, dry mouth and a sore throat for 6 weeks following his return from a family trip abroad. He did not have any significant medical history and did not take any regular medicines. There were no previous blood tests recorded.
Investigations
Case 1
The patient’s estimated glomerular filtration rate (eGFR, calculated by Modification of Diet in Renal Disease (MDRD) equation) had deteriorated from 37 to 31 mL/min over a 3-month period with no historical blood tests prior to this. Serum albumin was 44 g/L, adjusted calcium 2.34 mmol/L and haemoglobin 13.7 g/dL. Serum protein electrophoresis (SPE) showed an immunoparesis with a suppressed IgA and IgM at 0.67 and 0.4 g/L, respectively, but no paraprotein band was detected.
Urinalysis revealed blood and protein with a urine protein:creatinine ratio (UPCR) of 71 mg/mmol. Urinary free light chains (Bence Jones proteins) were detected by immunofixation.
sFLC analysis showed a raised serum free κ concentration of 787 mg/L giving a κ:λ ratio of 40.6.
A renal biopsy was undertaken. This showed mesangial hypercellularity (figure 1) with well-developed light chain deposition disease but no evidence of cast nephropathy.
Figure 1.
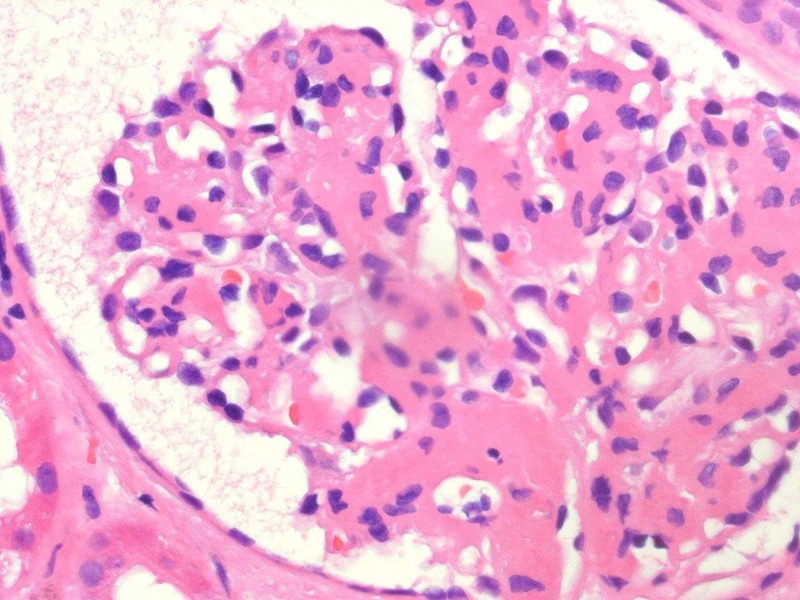
The mesangial hypercellularity seen by light microscopy when examining the native renal biopsy from case 1.
Bone marrow aspiration and trephine examination showed 20–25% plasma cell infiltration with expression of nuclear cyclin D1 and κ light chain restriction, consistent with a diagnosis of plasma cell dyscrasia.
Case 2
The patient had a decline in eGFR from 55 to 29 mL/min over a 2-month period. Serum albumin was 31 g/L, adjusted calcium 2.36 mmol/L and haemoglobin 9.1 g/dL (normocytic). SPE showed raised IgG concentration at 18.4 g/L but no paraprotein band and no evidence of immunoparesis (IgM 3.7 g/L, IgA 1.6 g/L).
Urinalysis was positive for protein and blood with a UPCR of 166 mg/mmol. Urinary free light chains were detected by immunofixation.
sFLC analysis performed as a consequence of the urine results showed serum free κ and λ concentrations were 2790 and 105 mg/L, respectively, giving a κ:λ ratio of 26.6.
A renal biopsy showed marked background chronic tubulointerstitial damage and mild arteriosclerosis. No evidence of myeloma casts or monoclonal immunoglobulin deposition disease was seen.
Bone marrow aspiration and trephine examination showed 20% plasma cell infiltration with expression of CD138, CD56 and nuclear cyclin D1 and κ light chain restriction, consistent with a diagnosis of plasma cell dyscrasia.
Case 3
The patient presented with an eGFR of 8 mL/min with no historical blood tests available for comparison. Serum albumin was 37 g/L, adjusted calcium 1.96 mmol/L (parathyroid hormone 319 ng/L) and haemoglobin 7.7 g/dL (normocytic). SPE did not reveal a paraprotein band or immunoparesis.
Urinalysis showed blood and protein with a UPCR of 178 mg/mmol. Urine was not analysed for free light chains.
sFLC analysis was performed which showed serum free κ and λ concentrations of 1130 and 66 mg/L, respectively, giving a κ:λ ratio of 17.1.
A renal biopsy was undertaken which showed nodular glomerulosclerosis, with evidence of κ light chain restriction on immunohistochemistry and deposition of dense, granular material on ultrastructural examination. These findings were in keeping with light chain deposition disease. There was also marked background chronic tubulointerstitial damage.
Examination of the bone marrow showed 15% plasma cell infiltration. The bone marrow was hypercellular owing to an infiltrate of CD138, CD56 and cyclin D1 positive plasma cells that did not express CD20 and were clearly κ light chain restricted. These findings were consistent with plasma cell dyscrasia.
Treatment
All three patients were subsequently reviewed by the haematology department and have been started on chemotherapy. A combination of thalidomide and dexamethasone was used in case 1 (cyclophosphamide was not used due to renal impairment) and a combination dexamethasone and bortezomib were used in cases 2 and 3.
Outcome and follow-up
Two of the patients have advanced chronic kidney disease and one is dialysis dependent.
Discussion
Plasma cell dyscrasias are characterised by the proliferation of a clone of B-cell lineage. Plasma cells arise from B cells in the bone marrow and produce immunoglobulins that constitute the body's normal humoral immune response. Immunoglobulins are modular proteins, consisting heavy chains and light chains. In the monomeric IgG form, two heavy chains and two light chains are bound by disulfide bonds. During normal immunoglobulin production, light chains are produced in excess of heavy chains, with the surplus being released into the circulation as free light chains. These free light chains are primarily cleared from the circulation in the kidneys. Being low-molecular-weight proteins, they are relatively freely filtered at the glomerulus and are vigorously reabsorbed into proximal tubule epithelial cells, where they undergo hydrolysis, after which the constituent amino acids and peptides are returned to the circulation. In plasma cell dyscrasias, each abnormally expanded clone of malignant plasma cells produces an excess of either intact immunoglobulin or free light chains of a single type called a monoclonal protein (M-protein) or paraprotein.5 It is well recognised that kidney damage is a feature of these diseases, ranging from slowly progressive kidney disease to life-threatening acute kidney injury.6 There are a variety of laboratory tests used to diagnose plasma cell dyscrasias with the current British Society for Haematology guidelines recommending electrophoresis of serum and concentrated urine, followed by immunofixation to confirm and type any M-protein present. Immunofixation and sFLC assessment is indicated in patients where there is a strong suspicion of myeloma but in whom routine SPE is negative.7 Often urine testing can be unreliable, with samples failing to be sent from the outpatient area to the laboratory or incorrect specimen handling, resulting in delayed diagnosis. The cases presented highlight the importance of completing both aspects of the screening test, as had only SPE been carried out the diagnoses may not have been made.
SPE is the main screening tool for the assessment of intact monoclonal immunoglobulins, but its sensitivity may limit its utility for sFLC detection. Serum immunofixation electrophoresis is more sensitive for light chain detection but does not allow quatification.8 There is increasing evidence emerging that sFLC measurement is more sensitive than urine testing. It also has the advantages of being quantitative and accurate for free light chain concentrations. It requires only a single sample to be taken.9–11 As it is a nephelometric assay, sFLC results may be obtained quickly within a few hours, compared to electrophoresis and immunofixation assays. One further problem with measurement of urinary light chains is that dipstick tests for detecting proteinuria are unreliable in recognising free light chains and that light chain only disease may not cause hyperalbuminuria because glomerular function is intact. For free light chains to appear in the urine, the reabsorptive capacity of the proximal tubule has to be exceeded and so patients with sFLCs at lower concentrations may not have detectable urinary free light chains. Twenty-four hour urine collections have traditionally been used for the detection of light chains in patients with suspected MM; however, these are cumbersome and are prone to inaccuracies because of incorrect collection.12 Taggart et al suggest that the combination of SPE and sFLC analysis is the most clinically effective first-line diagnostic testing strategy for detecting plasma cell disorders.11 The International Myeloma Working Group (IMWG) has already dispensed with urinary testing, stating in the context of screening, the sFLC assay in combination with SPE and immunofixation yields high sensitivity and negates the need for 24 h urine studies for diagnoses, other than light chain amyloidosis (AL).13 It is important to note that when using the κ:λ light chain ratio in advanced kidney disease an adjustment in the acceptable range may be required from 0.26–1.65 to 0.37–3.1. This takes into account the reduction in sFLC clearance by the impaired kidneys, particularly the smaller κ molecules which are cleared faster in a normal functioning kidney.14 Hutchinson et al showed that when this altered ratio is employed, free light chain assays have a greater utility for identifying monoclonal FLC production than urine assessment in renal impairment.15
This case series highlights the importance of thorough and robust screening for MM in patients presenting with renal disease. An index of suspicion should be maintained regardless of the presence of proteinuria. We reiterate the need to screen patients for MM with a combination of serum electrophoresis/immunofixation and either urinary free light chain testing or sFLC measurement. We discuss the emerging role of the sFLC assay and the importance of sFLC measurement when screening for MM in patients with renal impairment.
Learning points.
Twenty per cent of multiple myeloma is light chain only disease which may not be identified with serum protein electrophoresis alone.
Serum protein electrophoresis/immunofixation and either urine free light chain testing or serum free light chain assay is required to screen for myeloma.
Serum free light chain assay is emerging as a useful screening tool for myeloma with several advantages over urinary testing.
Serum free light chain assay should be tested in anyone where either non-secretory or light chain only myeloma is suspected. This includes patients with an immunoparesis on serum protein electrophoresis. It should be considered in all patients presenting with renal impairment where screening for multiple myeloma is undertaken.
Adjustment must be made to the acceptable range for κ:λ ratio in advanced kidney disease.
Footnotes
Contributors: The clinical information was gathered by retrospective review of patient notes, a literary search was then conducted and the case report written by BT. The clinical information has been reviewed by KB and the histopathology by DW. The completed case report has been reviewed by all.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Katzel JA, Hari P, Vesole DH. Multiple myeloma: charging toward a bright future. CA Cancer J Clin 2007;57:301–18 [DOI] [PubMed] [Google Scholar]
- 2.Wikilite. Multiple myeloma—introduction. http://www.wikilite.com/wiki/index.php/Multiple_myeloma_-_Introduction
- 3.Prasad A, Abraham G. Acute renal failure, recovery in a patient with light chain myeloma and? Thalassemia trait. Indian J Nephrol 2005;15:243–4 [Google Scholar]
- 4.Knudsen LM, Hippe E, Hjorth M, et al. Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol 1994;53:207–12 [DOI] [PubMed] [Google Scholar]
- 5.Rao M, Lamont JL, Chan J, et al. Serum free light chain analysis for the diagnosis, management, and prognosis of plasma cell dyscrasias: future research needs: identification of future research needs from comparative effectiveness review no. 73. Rockville, MD: ): Agency for Healthcare Research and Quality (US), 2012. Report No:12-EHC135-EF [Google Scholar]
- 6.Basnayake K, Stringer SJ, Hutchison CA, et al. The biology of immunoglobulin free light chains and kidney injury. Kidney Int 2011;79:1289–301 [DOI] [PubMed] [Google Scholar]
- 7.Bird J, Owen R, D'Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011;154:32–75 [DOI] [PubMed] [Google Scholar]
- 8.Hutchison C, Basnayake K, Cockwell P. Serum free light chain assessment in monoclonal gammopathy and kidney disease. Nat Rev Nephrol 2009;5:621–8 [DOI] [PubMed] [Google Scholar]
- 9.Bradwell AR, Carr-Smith HD, Mead GP, et al. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003;361:489–91 [DOI] [PubMed] [Google Scholar]
- 10.Nowrousian MR, Brandhorst D, Sammet C, et al. Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res 2005;11(24 Pt 1):8706–14 [DOI] [PubMed] [Google Scholar]
- 11.McTaggart M, Lindsay J, Kearney E. Replacing urine protein electrophoresis with serum free light chain analysis as a first-line test for detecting plasma cell disorders offers increased diagnostic accuracy and potential health benefit to patients. Am J Clin Pathol 2013;140:890–7 [DOI] [PubMed] [Google Scholar]
- 12.Abraham R, Clark R, Bryant S, et al. Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chemistry 2002;48:655–7 [PubMed] [Google Scholar]
- 13.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 2009;23:215–24 [DOI] [PubMed] [Google Scholar]
- 14.Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1684–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


