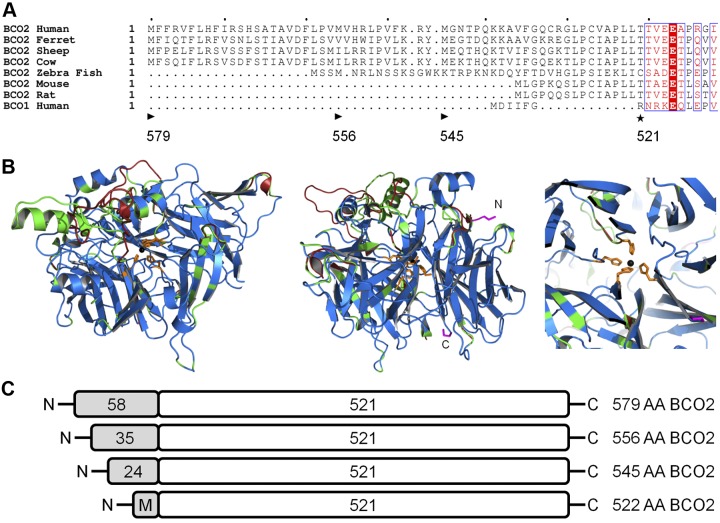
Figure 2.
Scheme for different human BCO2 variants. Three isoforms of human BCO2 have been previously identified that have overall sequence identity but differ at the length of their N termini. A) Alignment of the N-terminal sequence of BCO2 from several species, including the 579-aa (NP_114144.4), 556-aa (ACA05951.1), and 545-aa (NP_001032367.2) isoforms of human BCO2, as compared to human BCO1. Computational analysis predicts (P>0.989) a mitochondrial targeting sequence on the N terminus of the longest isoform. Arrowhead indicates the starting location of the different human isoforms; black asterisk indicates the putative proteolytic cleavage sites. Letters with a red background indicate sequence identity, red letters designate sequence similarity, and a period denotes a lack of homologous sequence. B) Overlay cartoon view of predicted human (modeled residues 54–578; green), mouse (modeled residues 10–531; red), and spatially aligned (blue) BCO2 structures using the SWISS-MODEL program based on template 3FSN model of related protein RPE65 (2.14 Å). Iron (black), chelating histidines (orange) and terminal residues (pink) are shown in detail. Helices and sheets point toward the C terminus. Left, the tunnel for RPE65 that leads to the active site iron is conserved in modeled murine and human BCO2 structures. Center, the two termini of BCO2 are visible on the surface. Right, the central chelating histidines are shown in detail. No difference in relative position was predicted for the active site histidines. C) Scheme of the different human BCO2 isoforms that were employed in this study. Note that the mitochondrial targeting sequence is lacking in the 522-aa BCO2 isoform, and a new translational start site was introduced by mutagenesis for this study.