Table 1.
Identification of the most active catalyst for the asymmetric α-alkylation of 7 and of the optimum reaction conditions
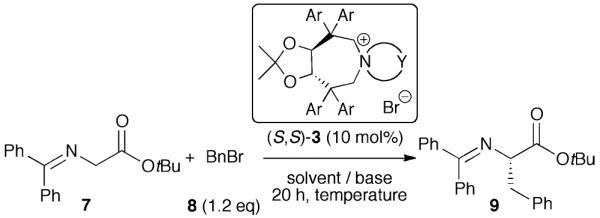
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Entry | Cat. | Ar | Ya | Solvent | Base | T/°C | Yieldb (Conv.)c (%) | ee (%)d (Conf.)e |
| 1 | 3aa | Ph | Aa | PhMe | KOH (50%) | rt | 70 (~90) | 34 (S) |
| 2 | −20 | 55 (~70) | 45 (S) | |||||
| 3 | 3ba | 4-tBu-Ph | 30(~50) | 61 (S) | ||||
| 4 | 3ca | 4-F-Ph | 35 (~50) | 34 (S) | ||||
| 5 | 3da | 3-Me-Ph | 77 (~90) | 55 (S) | ||||
| 6 | 3ea | 3,5-Me2-Ph | 47 (~60) | 5 (S) | ||||
| 7 | 3fa | m-Biphenyl | 45 (~60) | 7 (S) | ||||
| 8 | 3ga | p-Biphenyl | 65 (~80) | 79 (S) | ||||
| 9 | 3gb | Ba | 57 (~80) | 51 (S) | ||||
| 10 | 3gc | Ca | 21 (~30) | 25 (R) | ||||
| 11 | 3ga | p-Biphenyl | Aa | PhMe | CsOH·H2O | −70 | 54 (~70) | 39 (S) |
| 12 | CH2Cl2 | −70 | 35 (~50) | 38 (R) | ||||
| 13 | KOH (50%) | −20 | 53 (~70) | 28 (R) | ||||
| 14 | THF | 71 (~90) | 7 (S) | |||||
| 15 | PhF | 82 (>90) | 68 (S) | |||||
| 16 | benzene: PhMe (2:1) | 69 (~80) | 82 (S) | |||||
| 17f | 37 (~50) | 57 (S) | ||||||
| 18 | NaOH (50%) | 33 (~50) | 19 (S) | |||||
| 19 | CsOH (50%) | 24 (~40) | 65 (S) | |||||
| 20 | benzene : PhMe (1 : 1) | KOH (50%) | −35 | 61 (~75) | 84 (S) | |||
| 21 | PhMe | 54 (~70) | 87 (S) | |||||
| 22g | 81 (quant) | 87 (S) | ||||||
