Abstract
A high prevalence of the rtI187V polymerase substitution of hepatitis B virus (HBV) was detected in nucleoside/nucleotide-analogue-naive and -treated chronic hepatitis B (CHB) patients. We aimed at assessing the replicative capacity and susceptibility to lamivudine (LAM) and adefovir (ADV) in vitro of HBV harbouring rtI187V alone or in conjunction with LAM- or ADV-resistant mutations. The reverse transcriptase region of HBV isolates was directly sequenced from a cohort of 300 CHB patients from China. Replication-competent HBV constructs containing rtI187V and combined with LAM-resistant (rtM204I, rtL180M/rtM204V) mutations were generated, and compared with WT, LAM-resistant single (rtM204I) or double (rtL180M/rtM204V) and ADV-resistant (rtN236T) clones. In a Chinese cohort of 300 CHB patients, 8.7 % (26/300) showed substitution of rtI187 with V. Of note, the rtI187V prevalence in HBV genotype B was significantly higher than that in HBV genotype C (95.2 vs 4.8 %). In vitro phenotypic assays showed that the viruses bearing the rtI187V substitution had impaired replication efficacy when compared with the WT and the virus carrying rtI187V combined with LAM-resistant single or double mutations showed even more significantly impaired replicative capacities. Furthermore, rtI187V HBV remained susceptible towards treatment with LAM or ADV in vitro whereas the combination of the rtI187V substitution with LAM-resistant mutations rendered HBV resistant to LAM but still sensitive to ADV. Our study revealed that the rtI187V substitution in the HBV polymerase frequently occurred in CHB patients, particularly those with HBV genotype B. However, the emergence of the rtI187V substitution significantly impaired viral replication but without affecting drug sensitivity in vitro.
Introduction
Hepatitis B virus (HBV) can cause infectious inflammatory illness. According to the most recent World Health Organization (WHO) estimate, approximately two billion people worldwide have been infected with HBV, and among them 240 million have long-term chronic liver infections. China is a highly endemic area; there are an estimated 93 million HBV carriers, and among them 30 million are chronic hepatitis B (CHB) patients (Lu & Zhuang, 2009). The current agents for the treatment of CHB in China include immunomodulatory agents, such as IFN-α and pegylated IFN-α, and oral antiviral agents, including lamivudine (LAM), adefovir (ADV), entecavir, telbivudine and tenofovir (TDV) (Lam et al., 2011; Min & Dienstag, 2007; Papatheodoridis et al., 2008). LAM, the first nucleoside analogue (NA), is still the first-line antiviral drug for treatment of CHB in China. However, the effectiveness of NAs is hampered by the development of mutations in the reverse transcriptase (RT) resulting in progressive liver disease. ADV is also effective against HBV and does not show cross-resistance to lamivudine. It is often used for the treatment of HBV patients resistant to LAM (Lada et al., 2004; Lim et al., 2012). Although LAM at the dosage used has higher incidence of creating drug resistance (HBV remains sensitive to ADV or TDV), it remains to be a popular antiviral drug in many parts of the world because of its lower cost, excellent safety profile in long-term use and absence of oncogenic potential in animal studies (Yeh et al., 2011). NAs inhibit HBV replication through their phosphorylated-metabolite action on HBV DNA polymerase (Krishnan et al., 2002). However, a long duration of NA therapy is associated with an increasing risk of development of drug resistance (Fung et al., 2011).
HBV is a small, relaxed-circular, partially dsDNA virus (3.2 kb). Due to lack of proofreading activity of viral RT, new virions possess diverse genetic variability (Doo & Liang, 2001; Malik et al., 2012; Suk et al., 2002). Evolution of mutations that confer a great degree of resistance and restore replication fitness can be associated with progression of liver disease (Yim et al., 2006; Zhong et al., 2012).
In this study, we analysed nucleotide and amino acid sequences of 300 CHB patients from China. We identified a relatively high prevalence of novel rtI187V substitution in the B domain of the RT found in both NA-naive and NA-treated patients. This caused amino acid substitution rtI187V, with nucleoside position 690 mutated from A to G and no amino acid changed at the corresponding overlapping S gene. We examined the impact of the rtI187V substitution of HBV polymerase on the replication efficacy as well as susceptibility to LAM and ADV in comparison with WT, LAM- and ADV-resistant viruses in vitro.
Results
High prevalence of rtI187V polymerase substitution in a large cohort of CHB patients
We analysed HBV isolates from a cohort of 300 CHB patients from China by direct sequencing of the polymerase region. Based on the comparison of amino acid sequences obtained in this study with corresponding genotype consensus reference sequences (GenBank accession numbers, genotype A: D00329, AF100309 and AB033554; genotype B: X04615, AY123041 and AB014381), we found a relatively high frequency of substitution at position rt187 from isoleucine to valine (rtI187V). In total, 8.7 % (26/300) showed substitutions in the rtI187V locus (22 males/4 females, 14 NA-naive, 4 LAM-treated, 2 ADV-treated, 3 sequential LAM- and ADV-treated, 3 LAM add-on ADV-treated).
Patients with rtI187V polymerase substitution did not display significant differences as compared with patients with WT HBV with respect to age and sex (Table 1). Moreover, rtI187V substitution showed no significant difference between NA-treated and NA-naive patients (53.8 % vs 46.2 %). Of note, rtI187V was found more frequently in HBV genotype B than HBV genotype C (92.3 % vs 7.7 %).
Table 1. Characteristics of patients with rtI187V-containing as well as LAM-resistant polymerase mutations.
| Characteristic | I187V | L180M/M204V(I)/I187V | M204(V)I/I187V | L180M/M204V(I) | M204V(I) |
| n (%) | 16 (5.3) | 6 (2) | 4 (1.3) | 29 (9.7) | 29(9.7) |
| Male/female (n) | 14/2 | 4/2 | 4/0 | 22/7 | 24/5 |
| Age, median (range) | 32 (17–61) | 33 (23–50) | 31 (29–40) | 33 (25–60) | 32.5 (9–58) |
| Current therapy (n, %) | |||||
| NA-naive | 14 (87.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LAM | 0 (0) | 1 (16.7) | 3 (75) | 17 (58.6) | 15 (51.7) |
| ADV | 2(12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sequential LAM and ADV | 0 (0) | 3 (50) | 0 (0) | 6 (20.7) | 5 (17.2) |
| LAM add-on ADV | 0 (0) | 2 (33.3) | 1 (25) | 6 (20.7) | 9 (31) |
| Genotype (n, %) | |||||
| B | 16 (100) | 4 (66.7) | 4 (100) | 18 (62.1) | 25 (86.2) |
| C | 0 (0) | 2 (33.3) | 0 (0) | 11 (37.9) | 4 (13.8) |
Influence of rtI187V substitution and drug-resistant mutations on intracellular HBV DNA replication efficacy
It has been demonstrated that mutations of the HBV polymerase impact susceptibility to NAs as well as viral replication fitness (Fu & Cheng, 1998; Malik et al., 2012; Tacke et al., 2004a). Here, we aimed to understand the impact of rtI187V substitution on viral replication. Replication-competent HBV constructs harbouring the rtI187V substitution were constructed and compared with WT as well as LAM- (rtM204I, rtM204V/rtL180M) and ADV-resistant (rtN236T) constructs. The intracellular replication efficacies of these constructs were analysed by isolating the HBV replicative intermediates. Briefly, intracellular HBV nucleocapsids were harvested from cell lysates 4 days after transient transfection of HepG2 cells, and intracellular replicative intermediates were isolated and detected by Southern blot. The amount of detected HBV replicative intermediates (Fig. 1a) was normalized to total protein content of cell lysates and β-galactosidase (β-Gal) transfection efficacy. The intensity of the signals was quantified by using Image J software and was normalized to the WT (Fig. 1b). The rtI187V mutant showed a reduced replication level when compared with the WT. In accordance with a previous study (Fu & Cheng, 1998), HBV DNA levels were significantly reduced in LAM-resistant mutations (rtM204I or rtM204V/rtL180M). However, combination of these mutations with rtI187V resulted in further reduced HBV intracellular DNA levels, especially in the rtL180M/rtM204V/rtI187V combined mutation, which could not be detected by Southern blot.
Fig. 1.
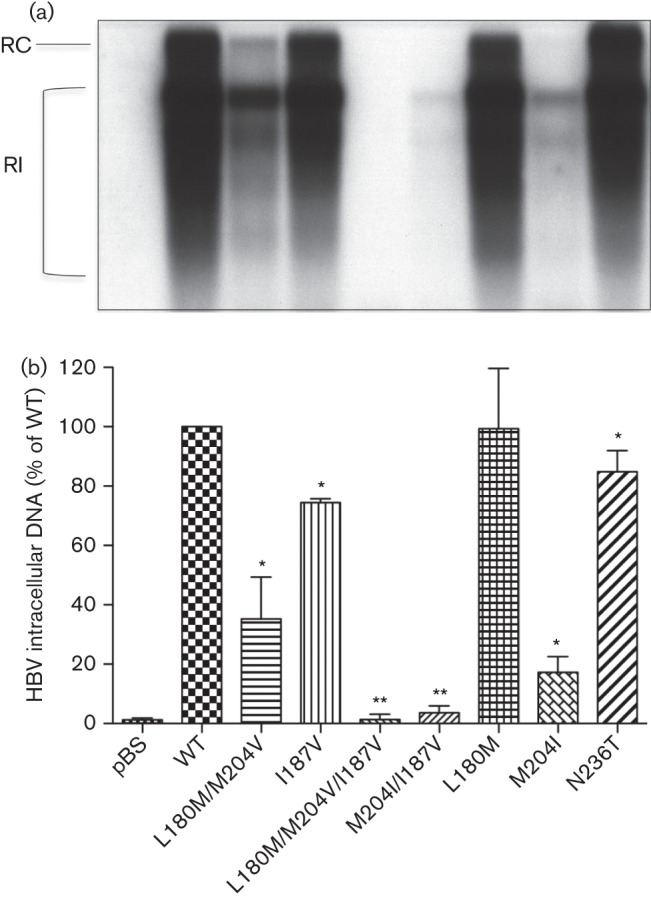
Intracellular replication capacity of HBV constructs. HepG2 cells were transiently transfected with replication-competent HBV constructs, and viral replication was assessed by HBV replicative intermediates after 4 days. Means and sd are given relative to WT. Experiments were performed in triplicate. (a) Intracellular replicative intermediates were analysed by Southern blot. (b) Quantification of HBV replicative intermediates. In comparison with WT, rtI187V and LAM-resistant mutations showed significantly impaired production of HBV replicative intermediates, while the reduction was even worse when rtI187V was introduced into HBV with LAM-resistant mutations. HBV replicative intermediates were quantified and normalized to the protein concentration of the cell lysates and β-Gal transfection efficiency. pBS, pBluescript vector; RC, relaxed circular; RI, replicative intermediates. *P<0.05, **P<0.01 (compared with WT).
Effect of rtI187V substitution and drug-resistant mutations on extracellular HBV virions and hepatitis B antigen secretion
Newly synthesized HBV virions released into the supernatant of transfected cells were analysed by quantitative real-time PCR. Tenfold serially diluted HBV WT plasmid of known concentration was used to generate a standard curve for quantitative PCR amplification. In line with the intracellular replication efficacies, reduced HBV extracellular virions were detected with rtI187V, ADV- and LAM-resistant constructs, and also with the combination of rtI187V and LAM-resistant mutations (Fig. 2a).
Fig. 2.
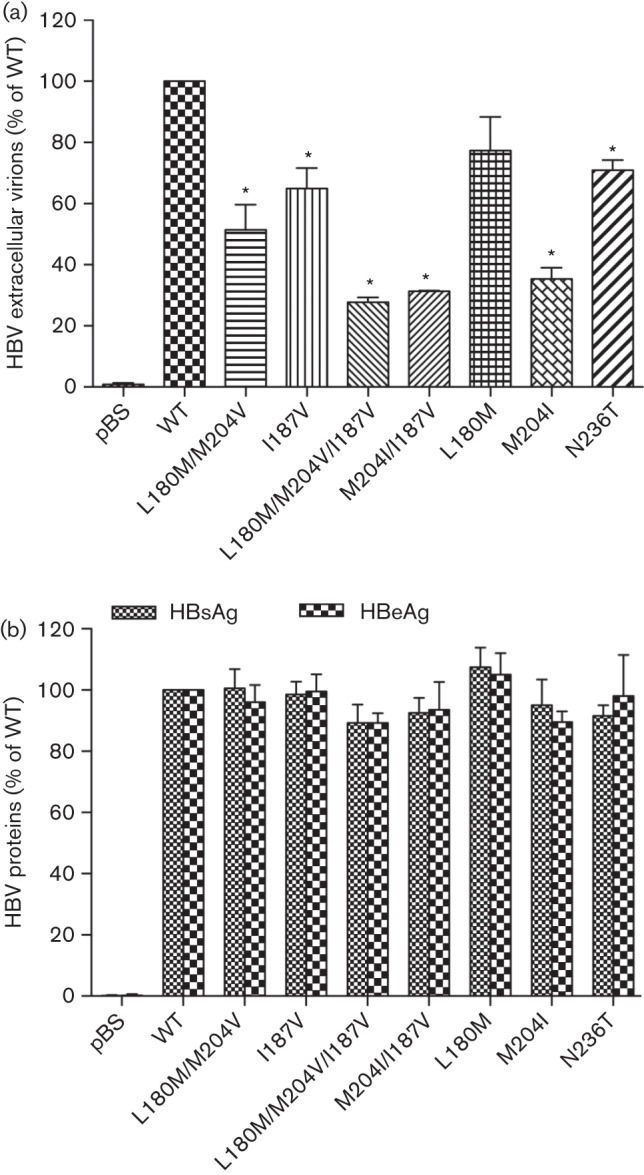
Release of HBV extracellular virions and proteins. HepG2 cells were transiently transfected with replication-competent HBV constructs, and extracellular release of HBV virions and protein was quantified from supernatant after 4 days. Means and sd are given relative to WT. Experiments were performed in triplicate. (a) HBV virions were quantified from culture supernatant by real-time PCR, and normalized to β-Gal transfection efficiency. In comparison with WT, LAM- and ADV-resistant mutants showed significantly reduced HBV copy numbers. Similarly, the copy number of HBV virions also decreased in rtI187V mutants and with the background of LAM-resistant mutations. (b) HBsAg and HBeAg concentrations were detected from supernatant by ELISA and normalized to transfection efficiency. pBS, pBluescript vector. *P<0.05 (compared with WT).
Furthermore, the rtI187V substitution did not affect the secretion of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in the supernatant (Fig. 2a). However, these data showed that the rtI187V substitution affected viral replication efficiency, especially when combined with LAM-resistant mutations.
RtI187V substitution did not confer resistance to LAM and ADV
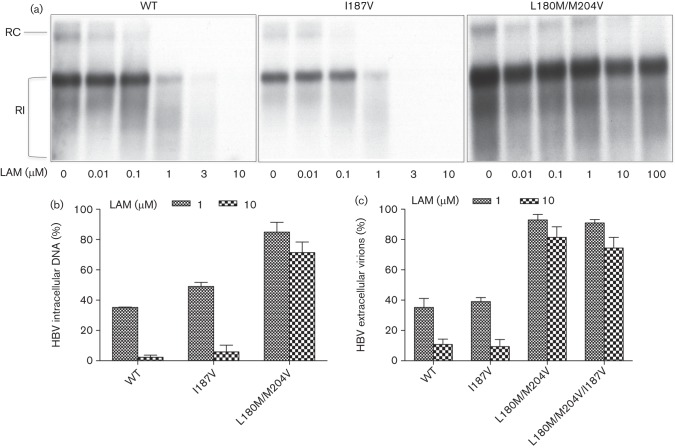
To test the susceptibility of HBV harbouring the rtI187V substitution to the antiviral drug LAM, cells were transiently transfected as described in Methods and kept in the presence of increasing concentrations of LAM or ADV for 4 days by quantifying HBV replicative intermediates using Southern blot analysis (Fig. 3a). Due to the markedly decreased replication efficacies conferred by the combination mutations (rtM204I/rtI187V, rtM204V/rtL180M/rtI187V), intracellular viral replication almost cannot be detected by Southern blot, so secreted HBV virions from supernatant were PEG-precipitated and measured by real-time PCR (Fig. 3c). As shown in Fig. 3(b, c), LAM significantly reduced the viral replication and HBV viral load of WT HBV and that carrying the rtI187V substitution. Whereas, the rtL180M/rtM204V construct conferred resistance to LAM. By contrast, introduction of the rtI187V substitution into a LAM-resistant (rtL180M/rtM204V) background did not affect the drug sensitivity of LAM-resistant mutations to LAM as quantified by HBV extracellular virions (Fig. 3c).
Fig. 3.
Sensitivity to LAM. (a) HepG2 cells were transiently transfected with HBV constructs as indicated. LAM was added to the culture medium at different concentrations as indicated. Replicative intermediates of HBV were determined as a measure of HBV replication. One representative experiment is shown. (b) Quantification of HBV replicative intermediates after incubation with 1 and 10 µM LAM. Means and sd are based on three independent experiments; values are given relative to incubation without LAM. (c) Quantification of extracellular HBV virions from supernatant 4 days after transient transfection of HBV constructs and after PEG precipitation of secreted virions; values are given relative to incubation without LAM. RC, Relaxed circular; RI, replicative intermediates.
Susceptibility of rt187 substitution and drug-resistant mutants to ADV in vitro
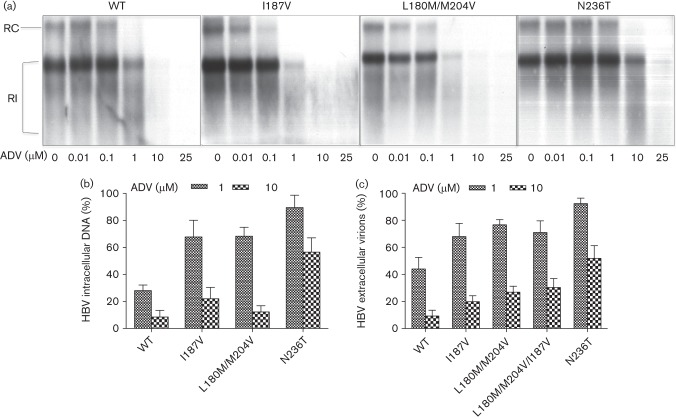
To test the susceptibility of the different HBV mutants to the antiviral drug ADV, cells were treated as described in detecting susceptibility to LAM. As shown in Fig. 4, all constructs except rtN236T were sensitive to ADV.
Fig. 4.
Sensitivity to ADV. (a) HepG2 cells were transiently transfected with HBV constructs as indicated. ADV was added to the culture medium at different concentrations as indicated. Replicative intermediates of HBV were determined as a measure of HBV replication. One representative experiment is shown. (b) Quantification of HBV replicative intermediates after incubation with 1 and 10 µM ADV. Means and sd are based on three independent experiments; values are given relative to incubation without ADV. (c) Quantification of extracellular HBV virions from supernatant 4 days after transient transfection of HBV constructs and after PEG precipitation of secreted virions; values are given relative to incubation without ADV. RC, Relaxed circular; RI, replicative intermediates.
Discussion
Available antiviral agents are effective in suppressing HBV replication with the advantage of delaying disease progression (Chae & Hann, 2007; Tillmann, 2007). However, high genetic variability confers a biological advantage, allowing HBV the ability to escape from the host’s antiviral immune responses and develop drug resistance (Melegari et al., 1998). Naturally occurring mutations of RT in treatment-naive patients have been reported in asymptomatic HBV carriers or CHB patients (Zheng et al., 2012). However, the impact of naturally occurring mutations in treatment-naive patients with CHB has not been fully evaluated. In this study, we report the prevalence and molecular and functional consequences of HBV variants harbouring the rtI187V substitution in 300 CHB patients. To our knowledge, this is the first time the impact of the rtI187V polymerase substitution on HBV replication efficiency has been addressed.
The rtI187V substitution was frequently detected in LAM-resistant patients. However, the relationship between rtI187V and LAM resistance has not been investigated. It is possible that the relatively high incidence of rtI187V substitutions in our cohort may be related to particularities of the patient population; all patients were recruited from southern China and 76.7 % (230/300) of the patients were infected by HBV genotype B. This is consistent with other studies that genotype B is most commonly found in southern China (Zhang et al., 2011). Moreover, the rtI187V substitution is found significantly more frequently in genotype B than in genotype C, which could be interpreted as certain patterns are restricted by functional constraints on a particular genotype (Warner et al., 2007). The genotype of HBV (B vs C) may play an important role in the prevalence of this substitution. Furthermore, our cell culture study also demonstrated that HBV isolates carrying the rtL180M/rtM204V mutation replicated better than those harbouring rtL180M/rtM204V/rtI187V. This suggested the possibility of better progression of LAM treatment in patients with the rtI187V substitution. A larger patient population study will be worthwhile in addressing the issue.
In this study, we also aimed to identify the impact of a high prevalence substitution, rtI187V, within the HBV polymerase region on viral replication capacity as well as antiviral drug susceptibility using HBV constructs. In vitro phenotypic studies showed that rtI187V, LAM- (rtM204I, rtM204V/rtL180M) and ADV-resistant (rtN236T) mutations conferred significantly impaired viral replication efficiencies while rtL180M conferred an equivalent replication capacity when compared with the WT, confirming previous results of other investigators (Amini-Bavil-Olyaee et al., 2009b; Brunelle et al., 2005; Fu & Cheng, 1998; Lada et al., 2004; Tacke et al., 2004b). However, the rtI187V substitution showed a stronger effect in decreasing the intracellular DNA replication efficiency conferred by LAM-resistant mutations. Drug sensitivity assays showed that rtI187V mutants remain susceptible to LAM as well as ADV, similar to the WT. Furthermore, isolates containing the rtI187V substitution together with LAM-resistant constructs (rtM204I, rtM204V/rtL180M) showed similar sensitivity to LAM and ADV as those harbouring LAM-resistant constructs. Our results demonstrated that the rtI187V mutation impairs viral replication efficiency without affecting susceptibility to LAM or ADV in vitro.
This study also found that, regardless of the selective pressure of drug, the replication of intracellular HBV DNA decreased significantly with either rtM204I/rtI187V or rtM204V/rtL180M/rtI187V. Therefore, it was easy to clear intracellular DNA during antiviral treatment in CHB patients with this kind of mutation.
Taken together, the presence of the rtI187V substitution confers the same sensitivity to LAM and ADV as the WT. Mutations related to LAM resistance could affect both genotype and susceptibility to LAM but have no impact on susceptibility to ADV. These observations may have important clinical implications; rtI187V HBV of patients treated by LAM or ADV may have less incidence or delayed onset of developing LAM resistance unless new compensatory mutants of RT occur, which was not been observed yet. If this is correct, surveillance of the rtI187V substitution of HBV may be useful for progression of LAM treatment. A large cohort study should be initiated to test this hypothesis.
Methods
Patient cohort.
Serum samples were obtained from a large cohort of 300 CHB patients from China that were recruited between 2008 and 2009. They were 85 % males and 15 % females with a median age of 33 years (range 9–70 years). The patients were grouped into five groups including NA-naive group (n = 169), LAM-treated group (n = 60), ADV-treated-group (n = 37), sequential LAM and ADV-treated group (n = 16) and LAM add-on ADV-treated group (treated with LAM and ADV due to the emergence of LAM resistance) (n = 18). No other NAs were administered. Exclusion criteria included hepatitis C, hepatitis D and human immunodeficiency virus co-infection. HBV DNA was extracted from serum samples and sequenced. Phylogenetic analysis revealed that 76.7 % patients were infected by genotype B HBV and 23.3 % were infected by genotype C HBV. All of the protocols for the collection and handling of patient samples and data were reviewed and approved by the ethics committee of the Shanghai Jiao Tong University School of Medicine, in compliance with the Helsinki Declaration of the World Medical Association. All samples were collected for routine clinical detection, with doctor’s prescription and patients’ informed consent.
Generation of HBV polymerase mutants.
The plasmid pHBV-adr encodes a WT, terminally redundant (1.1-genome-length), replication-competent HBV genome, which was a gift from Yuan Wang, Shanghai Institute of Biochemistry, Chinese Academy of Sciences (Fu & Cheng, 2000; Fu et al., 1996). It was used as a WT control and as a template in the site-directed mutagenesis performed with a Quick Change II site-directed mutagenesis kit (Stratagene). After generation of constructs, all were confirmed by digestion followed by sequencing the HBV RT region.
Cell culture and transfection.
Human hepatoblastoma HepG2 cells (from the American Type Culture Collection) were cultured in minimum essential medium (MEM, Gibco) supplemented with 10 % FBS and 100 U kanamycin ml−1. HepG2 cells were incubated in a humidified incubator at 37 °C with 5 % CO2. Six-well plates were seeded with 7×105 HepG2 cells per well a day earlier and transiently transfected by the replication-competent HBV plasmids (3 µg per well of six-well plate) using Lipofectamine 2000 following the manufacturer’s protocol (Invitrogen). The transfected cells were harvested after 4 days. pSV-β-Gal control vector (0.3 µg; Promega) was co-transfected to assess transfection efficiency and measurement of β-Gal activity from cell lysates as described by Bock et al. (2002). All experiments were performed three times.
Isolation of HBV intracellular replicative intermediates.
HBV intracellular replicative intermediates were isolated from transfected cells as described by Zhong et al. (2012). Briefly, cells were harvested 4 days after transfection and lysed with a buffer containing 1 % NP-40. Total protein content of the cell lysates was quantified using a Bio-Rad protein assay according to the instructions of the manufacturer. Cell lysates were centrifuged for 5 min at 10 000 g. Supernatants were treated with DNase I (100 µg ml−1) and RNase A (100 µg ml−1) (Roche) with 10 mM MgCl2, and incubated at 37 °C for 1.5 h to eliminate contaminating plasmid HBV DNA. After that, HBV capsids were digested by using 25 mM EDTA/100 mM NaCl/1 % SDS (w/v)/200 µg proteinase K ml−1, and incubated at 50 °C for 2 h, followed by phenol/chloroform extraction and precipitation with 2-propanol. Pellets containing viral DNA were dissolved in double distilled water before analysis by Southern blot.
Analysis of HBV intracellular replicative intermediates by Southern blot hybridization and quantification.
The intracellular HBV DNA replicative intermediate was detected by a full-length HBV DNA probe labelled with digoxigenin-dUTP (Roche). The signal intensity data were quantified using Image J software (Bio-Rad) and normalized to total protein content of the cell lysates and transfection efficiency.
Quantification of HBV extracellular virions by real-time PCR.
HBV viral particles were precipitated from the cell culture supernatant 4 days after transfection using polyethylene glycol 8000 (10 %, v/v) in 1.5 M NaCl at 4 °C overnight followed by DNase I and RNase A digestion (Roche). HBV DNA was extracted by proteinase K/SDS, phenol/chloroform extraction, and 2-propanol precipitation of mature HBV DNA, as described above. To determine HBV viral load in cell culture supernatant, a real-time PCR was carried out on a 157 bp fragment from a highly conserved region of the HBV-S gene amplified using SsoFast EvaGreen Supermix (Bio-Rad) (Amini-Bavil-Olyaee et al., 2009a). A standard curve was prepared using 10-fold serial dilutions of the HBV WT vector, and viral load was quantified by the Bio-Rad CFX connect real-time PCR machine. After quantification of HBV DNA copy numbers, the viral load was normalized to β-Gal transfection efficiency.
Detection of HBV protein in supernatant.
HBsAg and HBeAg in culture medium were quantified by ELISA (Abnova).
Antiviral compounds and drug susceptibility assays.
LAM was provided by Chung K. Chu from the University of Georgia. ADV was kindly provided by Gilead Sciences. Stock solutions for LAM (100 mM) and ADV (16.8 mM) were dissolved in sterile distilled water, aliquoted and stored frozen at −20 °C. The analyses of drug susceptibility were performed after transient transfection of HepG2 cells with recombinant WT and mutant clones. After 1 day, the medium containing various concentrations of each antiviral drug was changed every day and cells were harvested after 4 days. The concentration of LAM used was 0, 0.01, 0.1, 1, 3 and 10 µM for WT HBV, and 0, 0.01, 0.1, 1, 10 and 100 µM for HBV containing LAM-resistant mutations; the concentration of ADV used was 0, 0.01, 0.1, 1, 10 and 25 µM. The transfection efficiency was assessed by co-transfection of a plasmid encoding β-Gal performed and β-Gal enzyme assay (Promega). Southern blot results were normalized to the total protein content of the cell lysates and transfection efficiency based on β-Gal activity.
Statistical analysis.
Data are reported as means±sd. Groups’ comparisons were performed using Student’s t-test. All statistical analyses were carried out with SPSS software. A P value of less than 0.05 was considered statistically significant.
Acknowledgements
This research was supported by NIH grant RO1AI38204. Yung-Chi Cheng is a fellow of the National Foundation of Cancer Research. LAM was kindly provided by Chung K. Chu from the University of Georgia and ADV was kindly provided by Gilead Sciences.
References
- Amini-Bavil-Olyaee S., Herbers U., Sheldon J., Luedde T., Trautwein C., Tacke F. (2009a). The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positive and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology 49, 1158–1165. 10.1002/hep.22790 [DOI] [PubMed] [Google Scholar]
- Amini-Bavil-Olyaee S., Herbers U., Mohebbi S. R., Sabahi F., Zali M. R., Luedde T., Trautwein C., Tacke F. (2009b). Prevalence, viral replication efficiency and antiviral drug susceptibility of rtQ215 polymerase mutations within the hepatitis B virus genome. J Hepatol 51, 647–654. 10.1016/j.jhep.2009.04.022 [DOI] [PubMed] [Google Scholar]
- Bock C. T., Tillmann H. L., Torresi J., Klempnauer J., Locarnini S., Manns M. P., Trautwein C. (2002). Selection of hepatitis B virus polymerase mutants with enhanced replication by lamivudine treatment after liver transplantation. Gastroenterology 122, 264–273. 10.1053/gast.2002.31015 [DOI] [PubMed] [Google Scholar]
- Brunelle M. N., Jacquard A. C., Pichoud C., Durantel D., Carrouée-Durantel S., Villeneuve J. P., Trépo C., Zoulim F. (2005). Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology 41, 1391–1398. 10.1002/hep.20723 [DOI] [PubMed] [Google Scholar]
- Chae H. B., Hann H. W. (2007). Time for an active antiviral therapy for hepatitis B: an update on the management of hepatitis B virus infection. Ther Clin Risk Manag 3, 605–612. [PMC free article] [PubMed] [Google Scholar]
- Doo E., Liang T. J. (2001). Molecular anatomy and pathophysiologic implications of drug resistance in hepatitis B virus infection. Gastroenterology 120, 1000–1008. 10.1053/gast.2001.22454 [DOI] [PubMed] [Google Scholar]
- Fu L., Cheng Y. C. (1998). Role of additional mutations outside the YMDD motif of hepatitis B virus polymerase in L(-)SddC (3TC) resistance. Biochem Pharmacol 55, 1567–1572. 10.1016/S0006-2952(98)00050-1 [DOI] [PubMed] [Google Scholar]
- Fu L., Cheng Y. C. (2000). Characterization of novel human hepatoma cell lines with stable hepatitis B virus secretion for evaluating new compounds against lamivudine- and penciclovir-resistant virus. Antimicrob Agents Chemother 44, 3402–3407. 10.1128/AAC.44.12.3402-3407.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Wu X., Kong Y. Y., Wang Y. (1996). Regulation of hepatitis B virus gene expression by its two enhancers. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 28, 590–599. [PubMed] [Google Scholar]
- Fung J., Lai C. L., Seto W. K., Yuen M. F. (2011). Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J Antimicrob Chemother 66, 2715–2725. 10.1093/jac/dkr388 [DOI] [PubMed] [Google Scholar]
- Krishnan P., Liou J. Y., Cheng Y. C. (2002). Phosphorylation of pyrimidine l-deoxynucleoside analog diphosphates. Kinetics of phosphorylation and dephosphorylation of nucleoside analog diphosphates and triphosphates by 3-phosphoglycerate kinase. J Biol Chem 277, 31593–31600. 10.1074/jbc.M205115200 [DOI] [PubMed] [Google Scholar]
- Lada O., Benhamou Y., Cahour A., Katlama C., Poynard T., Thibault V. (2004). In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther 9, 353–363. [PubMed] [Google Scholar]
- Lam Y. F., Yuen M. F., Seto W. K., Lai C. L. (2011). Current antiviral therapy of chronic hepatitis B: efficacy and safety. Curr Hepat Rep 10, 235–243. 10.1007/s11901-011-0109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. S., Lee J. Y., Lee D., Shim J. H., Lee H. C., Lee Y. S., Suh D. J. (2012). Randomized trial of entecavir plus adefovir in patients with lamivudine-resistant chronic hepatitis B who show suboptimal response to lamivudine plus adefovir. Antimicrob Agents Chemother 56, 2941–2947. 10.1128/AAC.00338-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F. M., Zhuang H. (2009). Management of hepatitis B in China. Chin Med J (Engl) 122, 3–4. [PubMed] [Google Scholar]
- Malik A., Singhal D. K., Albanyan A., Husain S. A., Kar P. (2012). Hepatitis B virus gene mutations in liver diseases: a report from New Delhi. PLoS ONE 7, e39028. 10.1371/journal.pone.0039028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegari M., Scaglioni P. P., Wands J. R. (1998). Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology 27, 628–633. 10.1002/hep.510270243 [DOI] [PubMed] [Google Scholar]
- Min A. D., Dienstag J. L. (2007). Oral antivirals for chronic hepatitis B. Clin Liver Dis 11, 851–868, ix. 10.1016/j.cld.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Papatheodoridis G. V., Manolakopoulos S., Archimandritis A. J. (2008). Current treatment indications and strategies in chronic hepatitis B virus infection. World J Gastroenterol 14, 6902–6910. 10.3748/wjg.14.6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk F. M., Lin M. H., Newman M., Pan S., Chen S. H., Liu J. D., Shih C. (2002). Replication advantage and host factor-independent phenotypes attributable to a common naturally occurring capsid mutation (I97L) in human hepatitis B virus. J Virol 76, 12069–12077. 10.1128/JVI.76.23.12069-12077.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F., Manns M. P., Trautwein C. (2004a). Influence of mutations in the hepatitis B virus genome on virus replication and drug resistance–implications for novel antiviral strategies. Curr Med Chem 11, 2667–2677. 10.2174/0929867043364333 [DOI] [PubMed] [Google Scholar]
- Tacke F., Gehrke C., Luedde T., Heim A., Manns M. P., Trautwein C. (2004b). Basal core promoter and precore mutations in the hepatitis B virus genome enhance replication efficacy of lamivudine-resistant mutants. J Virol 78, 8524–8535. 10.1128/JVI.78.16.8524-8535.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann H. L. (2007). Antiviral therapy and resistance with hepatitis B virus infection. World J Gastroenterol 13, 125–140. 10.3748/wjg.v13.i1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N., Locarnini S., Kuiper M., Bartholomeusz A., Ayres A., Yuen L., Shaw T. (2007). The L80I substitution in the reverse transcriptase domain of the hepatitis B virus polymerase is associated with lamivudine resistance and enhanced viral replication in vitro. Antimicrob Agents Chemother 51, 2285–2292. 10.1128/AAC.01499-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. T., Chen T., Hsu C. W., Chen Y. C., Lai M. W., Liang K. H., Chen T. C. (2011). Emergence of the rtA181T/sW172* mutant increased the risk of hepatoma occurrence in patients with lamivudine-resistant chronic hepatitis B. BMC Cancer 11, 398. 10.1186/1471-2407-11-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H. J., Hussain M., Liu Y., Wong S. N., Fung S. K., Lok A. S. (2006). Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 44, 703–712. 10.1002/hep.21290 [DOI] [PubMed] [Google Scholar]
- Zhang A. M., Wang H. F., Wang H. B., Su H. B., Xin S. J., Hu J. H., You S. L. (2011). [Distribution and clinical significance of HBV genotypes in patients with HBV infection in 30 regions of China]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 25, 126–128. [PubMed] [Google Scholar]
- Zheng J., Zeng Z., Zhang D., Yu Y., Wang F., Pan C. Q. (2012). Prevalence and significance of hepatitis B reverse transcriptase mutants in different disease stages of untreated patients. Liver Int 32, 1535–1542. 10.1111/j.1478-3231.2012.02859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Lv J., Li J., Xing X., Zhu H., Su H., Chen L., Zhou X. (2012). Prevalence, virology and antiviral drugs susceptibility of hepatitis B virus rtN238H polymerase mutation from 1865 Chinese patients with chronic hepatitis B. Antiviral Res 93, 185–190. 10.1016/j.antiviral.2011.11.012 [DOI] [PubMed] [Google Scholar]