Abstract
Background
The ethnobotanical importance of Prosopis juliflora is well-known in the folkloric system of medicine for the treatment of various ailments. Although, the study related to the antibacterial potential of this plant, from Central India is scanty.
Material and methods
The in vitro antibacterial activity of Prosopis juliflora leaves collected from the local area was evaluated against ten bacterial type cultures by agar well diffusion assay. The crude extracts prepared by two methods separately with three different solvents were examined for the preliminary antibacterial activity and phytochemical screening, the results of which were used for the choice of solvent and mass extraction of crude extract. Solvent fractionation of crude extract was done employing two sets of solvents namely Set-PCE and Set-HDB which resulted in total, six organic and two aqueous fractions, which were finally subjected to antibacterial activities.
Results
Varying degrees of growth inhibition was shown by all the fractions against tested microorganisms. The highest antibacterial activity was observed in aqueous fractions as compared to solvent fractions.
Conclusion
Isolation and characterization of the bioactive components can be further done by systematic screening of the most active solvent fraction which could lead to the possible source of new antibacterial agents.
Keywords: antibacterial activity, herbal extract, phytochemical screening, solvent fractionation
Introduction
Prosopis juliflora belongs to the family Fabaceae and is commonly known as mesquite. It is a fast growing, thorny deciduous, drought-resistant plant and has a wide crown and deep-rooted. It is native to Central and South America - spreading from southern Mexico to Panama and from the Caribbean Islands to northern South America and an invader species in India that competes with the native species (Pasiecnik et al., 2001; Harris et al., 2003; Seetha Lakshmi et al., 2010). It grows in all kinds of soil conditions, including wastelands at altitudes ranging from 0 to 1,500 m above sea level (Pasiecnik et al., 2001).
This herb is well-known in the folkloric system of medicine because of its ethnobotanical importance. The plant has been reported to treat oral ailments like toothache (Hebbar et al., 2004). The leaves were used against asthma, bronchitis, conjunctivitis (Agra et al., 2008) as well as against skin diseases, blood and venereal diseases and act as an insecticide (Senthilkumar et al., 2009). The crude extracts of various parts and purified chemical components have been found to possess antimicrobial, insecticidal and different pharmacological activities (Malik and Kalidhar, 2005). The high-potential activity of these extracts compared to selective antibiotics lead to evaluate the new antimicrobial agents to fight against the drug-resistant pathogens (Navya et al., 2011). Moreover, the leaves were found to be effective in reduction or eradication of the phytopathogens like Xanthomonas campestris and Agrobacterium rhizogenes in an eco-friendly way (Sheikh et al., 2012). The alkaloids present in the plant leaves can also be used as a lead bio-pesticide in combating the diseases caused by several phytopathogens on cereal crops (Seetha Lakshmi et al., 2010). Recent studies showed that the plant can be used in various therapeutic applications because of its non-toxic effects ensuring its quality and safety and can be used in the formulation of several pharmacologically active compounds (Prabha et al., 2012).
The antibacterial activity of various solvent extracts of different parts and compounds isolated from P. juliflora are reported by many researchers (Sathiya and Muthuchelian, 2008; Seetha Lakshmi et al., 2010; Navya et al., 2011; Singh et al., 2011; Hari Prasad et al., 2011) from distinct regions of India, but the plant from Central India origin has not been considered until now. The metabolic rates and activities of the same species of plant may vary from region to region, so it is important to reveal the medicinal properties of the plants in this region which has a higher biodiversity as compared to the other Indian tropical regions. The results of these studies showed that this plant holds innumerable virtues for the habitual treatment of various maladies and has a wide range of applicability as indigenous medicine since time immemorial. To the best of our knowledge, none of the studies were performed on various solvent fractions obtained from solvent fractionation so far. Considering the above views, the present study was designed with the main objectives to evaluate precisely the antibacterial potential of P. juliflora prevalent in Central India with respect to choice of solvents for phytoconstituents extraction, phytochemical screening and solvent fractionation.
Materials and Methods
The leaves of Prosopis juliflora collected locally were washed, shade-dried, crushed to fine powder of 60-mesh size and stored at 8° C until further use. The bacterial cultures used for antibacterial activity assays were maintained on desired media agar slants, stored at 4° C and sub-cultured periodically. The phytoconstituents extraction was done by cold (infusion) (1:4 w/v) and hot (soxhlet) (1:24 w/v) solvent extraction separately with methanol, ethanol and butanol. The percentage yield of each extract was calculated and the dried extracts were stored airtight at 4° C for further use. The in vitro antibacterial activity of three hot and three cold extracts at 100 mg/ml was performed by agar well diffusion assay on Mueller Hinton Agar (MHA) medium. Chloramphenicol and Ampicillin at a concentration of 0.5 mg/ml were used as positive controls and blank Dimethyl Sulfoxide (DMSO) as a negative control. The experiment was performed in triplicate for each bacterial strain and the antibacterial activity of each extract was expressed in terms of the mean of the diameter of zone of growth inhibition in mm (Thakur et al., 2012).
The phytochemical screening of all the six crude extracts (cold and hot) was individually performed for the presence of biologically active compounds by the standard procedure. The presence of alkaloids was detected by Dragendorff's reagent and Mayer's reagent, whereas anthraquinones by Borntrager's test and cardiac glycosides by Keller-Kiliani test and Legal test. Flavonoids were identified by alkaline reagent test and Shinoda test and saponins by froth test. Tannins were spotted by ferric chloride test and lead acetate test whereas terpenoids by Salkowski's test (Singh, 2012; De et al., 2010).
On the basis of the results of preliminary analyses of antibacterial activity and phytochemical screening, 200 g of fine leaf powder was soxhlet extracted with 1000 ml of methanol to obtain the crude methanolic extract. It was dissolved in 20% methanolic water to make a crude extract solution (CES) for solvent fractionation employing two sets of solvents namely Set-PCE (Petroleum spirit, Chloroform and Ethyl acetate) and Set-HDB (Hexane, Dichloromethane and Butanol) which resulted in total, six organic and two aqueous fractions viz. petroleum spirit fraction (PF), chloroform fraction (CF), ethyl acetate fraction (EF), hexane fraction (HF), dichloromethane fraction (DF), butanol fraction (BF) and remaining aqueous fraction (AF-I and AF-II), respectively. The percentage yield of each dried organic fractions (PF, CF, EF, HF, DF and BF) and aqueous fractions (AF-I and AF-II) was calculated and subjected to in vitro antibacterial activities at 25 mg/ml concentration by agar well diffusion assay on the MHA medium as described earlier (Thakur et al., 2012).
Results and Discussion
One kg fresh leaves of Prosopis juliflora after washing and drying were powdered and sieved which resulted in 400 g fine powder. The fine leaf powder was processed further for studies on antibacterial activity and phytochemical screening under laboratory conditions. The study of bacteria, in a laboratory with limited resources, often involves the use of living cultures. These need to be kept viable at least during experimental studies and if they prove of importance, they are to be maintained alive for future work. The primary aim was to maintain all the test bacteria in a viable state without morphological, physiological or genetic change until they are required for further use. The percentage yield of crude extracts after cold (infusion) and hot (soxhlet) extraction methods were calculated. The percentage yield of ethanol extract was the highest (16.47%) followed by methanol extract (12.76%) and butanol extract (6.61%) in case of cold extraction. In contrast, with hot extraction, butanol extract resulted in the highest percentage yield (55.04%), followed by ethanol extract (46.98%) and methanol extract (25.25%) as shown in Table 1.
Table 1.
Yield of herbal extracts obtained with three different solvents by two extraction methods
| Extraction method |
Solvent | Solvent volume (ml) |
Weight of powder (g) |
Weight of extract (g) |
% yield |
| Infusion (Cold) | Butanol | 40 | 8 | 0.529 | 06.61 |
| Ethanol | 40 | 8 | 1.318 | 16.47 | |
| Methanol | 40 | 8 | 1.021 | 2.76 | |
| Soxhlet (Hot) | Butanol | 240 | 10 | 5.504 | 55.04 |
| Ethanol | 240 | 10 | 4.698 | 46.98 | |
| Methanol | 240 | 10 | 2.525 | 25.25 |
In total, the percentage yield of extracts obtained from three different solvents by soxhlet extraction was higher than the yield obtained with infusion method. This finding correlates with our previous finding where soxhlet extraction of Sphaeranthus indicus leaves resulted in better yield when compared with cold infusion extraction method (Thakur et al., 2012). In an another study, the methanol extract of leaves of Annona squamosa resulted in similar percentage yield of 12.9% employing the cold maceration process (Patel and Kumar, 2008) while, the stem bark of Croton zambesicus was extracted with methanol to give a yield of 52.02 g equivalent to 12.64% using the soxhlet extractor (Reuben et al., 2008). The methanol extract from 750 g of the powdered bark of Afzelia africana resulted in 14% yield of 105 g employing cold extraction method (Akinpelu et al., 2009).
All the six crude extracts (three hot and three cold) were subjected to in vitro preliminary antibacterial bioassay against aforementioned ten different bacteria. The results showed that, cold and hot extracts at 100 mg/ml concentration significantly inhibited the growth of all test bacteria viz., Bacillus subtilis, Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Salmonella typhi and Salmonella typhimurium (Table 2). Overall, 100% bacteria produced the zone of inhibition during the screening process showing appreciable inhibitory effect. Control experiments of solvent (DMSO) used for the extract preparation showed no inhibition of any bacteria, indicating that the plant extract itself and not solvent inhibited the growth of the test bacteria (Fig 1, Plate 3, Well A, B). Chloramphenicol and Ampicillin showed variable inhibition diameters against gram-positive and gram-negative bacteria (Table 3).
Table 2.
Diameter of zone of growth inhibition in mm of antibacterial activity of alcoholic extracts prepared by two extraction methods
| Bacterial cultures | Herbal extract (100 mg/ml) | |||||
| Butanolic | Ethanolic | Methanolic | ||||
| Colda | Hotb | Colda | Hotb | Colda | Hotb | |
| B. subtilis | 23.33 ± 0.58 | 14.00 ± 0.00 | 17.33 ± 0.58 | 16.67 ± 0.58 | 17.67 ± 0.58 | 17.33 ± 0.58 |
| E. faecalis | 11.17 ± 0.29 | 12.33 ± 0.58 | 16.00 ± 0.00 | 06.67 ± 0.29 | 16.33 ± 0.58 | 18.00 ± 0.00 |
| E. coli | 17.00 ± 0.00 | 10.00 ± 1.00 | 17.00 ± 0.00 | 16.83 ± 0.76 | 15.33 ± 0.58 | 14.50 ± 0.50 |
| K. pneumoniae | 09.17 ± 0.29 | 05.00 ± 0.00 | 11.33 ± 0.58 | 06.33 ± 0.58 | 11.17 ± 0.29 | 10.00 ± 0.87 |
| P. aeruginosa | 17.33 ± 0.58 | 14.33 ± 0.29 | 16.00 ± 0.00 | 17.00 ± 0.00 | 22.33 ± 0.58 | 16.00 ± 0.00 |
| S. typhi | 15.00 ± 0.00 | 14.33 ± 0.58 | 12.67 ± 0.58 | 15.00 ± 0.00 | 18.33 ± 0.58 | 18.00 ± 0.00 |
| S. typhimurium | 05.67 ± 0.76 | 07.67 ± 0.58 | 06.33 ± 0.58 | 05.67 ± 0.58 | 07.00 ± 0.00 | 07.50 ± 0.50 |
| S. aureus | 05.33 ± 0.58 | 07.33 ± 0.58 | 06.67 ± 0.58 | 07.00 ± 1.00 | 08.00 ± 0.00 | 07.33 ± 0.58 |
| S. epidermidis | 20.17 ± 0.29 | 16.33 ± 0.58 | 17.00 ± 0.00 | 19.00 ± 0.00 | 21.00 ± 0.00 | 21.00 ± 0.00 |
| S. pyogenes | 24.83 ± 0.00 | 24.67 ± 0.58 | 30.00 ± 1.00 | 28.83 ± 0.29 | 30.00 ± 0.00 | 23.33 ± 0.58 |
Antibacterial activity was expressed in terms of the diameter of zone of growth inhibition (mean ± S.D., n = 3)
a: Infusion (cold extraction), b: Soxhlet (hot percolation)
Figure 1.
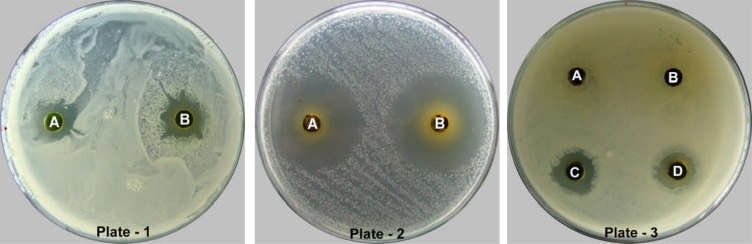
Showing antibacterial activity of crude herbal extract against Staphylococcus aureus (Plate-1); Streptococcus pyogenes (Plate-2) and Klebsiella pneumoniae (Plate-3).
Table 3.
Diameter of zone of growth inhibition in mm of antibacterial activity of standard drugs
| Bacterial cultures | Standard antibiotic (0.5 mg/ml) | |
| Chloramphenicol | Ampicillin | |
| B. subtilis | 23.17 ± 0.58 | 07.67 ± 0.29 |
| E. faecalis | 16.67 ± 0.29 | 27.50 ± 0.50 |
| E. coli | 27.17 ± 0.29 | 19.83 ± 0.29 |
| K. pneumoniae | 18.67 ± 0.29 | 06.33 ± 0.29 |
| P. aeruginosa | 07.50 ± 0.50 | 05.17 ± 0.29 |
| S. typhi | 26.83 ± 0.29 | 29.83 ± 0.29 |
| S. typhimurium | 17.83 ± 0.29 | 06.17 ± 0.29 |
| S. aureus | 19.67 ± 0.29 | 10.33 ± 0.29 |
| S. epidermidis | 24.83 ± 0.76 | 16.83 ± 0.29 |
| S. pyogenes | 28.50 ± 0.50 | 44.50 ± 0.50 |
Antibacterial activity was expressed in terms of the diameter of zone of growth inhibition (mean ± S.D., n = 3)
All the cold solvent extracts viz., butanolic, methanolic and ethanolic extracts exhibited the highest value of zone of inhibition against S. pyogenes giving zone of diameter of 24.83 ± 0.76 mm, 30.00 ± 1.00 mm and 30.00 ± 0.00 mm, respectively. Butanolic extract exhibited the lowest value of inhibition zone against S. aureus with 5.33 ± 0.58 mm zone of inhibition (Fig 1, Plate 1, Well A). Moreover, the least activity was observed against S. typhimurium showing 6.33 ± 0.58 mm and 7.00 ± 0.00 mm zone of inhibition, respectively for ethanolic and methanolic extracts (Table 2). Out of three cold extracts, ethanolic and methanolic extract displayed the highest inhibition zone of 30.00 ± 1.00 mm (Fig 1, Plate 2, Well A) and 30.00 ± 0.00 mm, respectively, against S. pyogenes whereas the lowest inhibition zone of 5.33 ± 0.58 mm was shown by butanolic extract against S. aureus.
In an another study, the cold ethanolic extract at 100 mg/ml showed a strong antibacterial effect on E. coli (12.81 ± 0.45 mm), S. aureus (12.72 ± 0.67 mm), Klebsiella sp. (11.83 ± 0.88 mm) and Salmonella sp. (11.04 ± 0.33 mm) using disc diffusion method (Singh et al., 2011) and the aqueous leaf extract at 1 g/ml had significant inhibitory activity against S. aureus and E. faecalis with 10.16 ± 0.28 and 12.20 ± 0.20 mm zone of inhibition (Hari Prasad et al., 2011). The extract also showed better activity than the commercially available mouth rinses against selected microbes which pre-dominate the oral and periodontal tissues. Similarly, the considerable inhibitory activity of the methanolic leaf extract was observed against E. coli and B. subtilis with 24 mm and 17 mm zone of inhibition at 15 mg/ml, respectively (Napar et al., 2012).
In case of hot solvent extracts, our data showed that the highest values of the diameter of zone of inhibition were exhibited by all three extracts viz., butanolic, methanolic and ethanolic extracts against S. pyogenes giving zone of 24.67 ± 0.58 mm, 28.83 ± 0.29 mm and 23.33 ± 0.58 mm, respectively. The lowest antibacterial responses of butanolic, ethanolic and methanolic extracts was observed against K. pneumoniae with 5.00 ± 0.00 mm, S. typhimurium with 5.67 ± 0.58 mm and S. aureus with 7.33 ± 0.58 mm zone of inhibition, respectively (Table 2). In all, the extensive inhibition zone of 28.83 ± 0.29 mm was presented by ethanolic extract against S. pyogenes (Fig 1, Plate 2, Well B) whereas the butanolic extract represented the lowest value of the inhibition zone of 5.00 ± 0.00 mm against K. pneumoniae (Fig 1, Plate 3, Well D).
The results are in fair correlation with the earlier study in which the ethanolic extract prepared by soxhlet extraction showed notable antibacterial activity against S. epidermidis (11.05 ± 0.47 mm), S. aureus (12.20 ± 0.76 mm), Streptococcus sp. (11.80 ± 0.44 mm), B. subtilis (10.78 ± 0.77 mm), S. typhimurium (11.15 ± 0.67 mm), P. aeruginosa (11.50 ± 0.55 mm), K. pneumoniae (12.10 ± 0.66 mm) and E. coli (11.70 ± 0.54 mm) at 100 mg/ml concentration using the agar disc diffusion assay (Sathiya and Muthuchelian, 2008). Alternatively, the methanolic extracts prepared by soxhlet extraction was effective against B. subtilis displaying 23.00 mm zone of inhibition at 100 mg/ml whereas compounds identified by the spectral data such as prosopidione, secojuliprosopinal and juliprosopine have shown the broad spectrum of antimicrobial activity with 14.00 mm, 13.00 mm and 12.00 mm inhibition zone at 1 mg/ml concentration (Seetha Lakshmi et al., 2010).
For the phytochemical screening, all the hot and cold crude extracts were analysed for the presence of secondary metabolites by specific reactions and identified by observing the intensity of colour developed and/or the appearance of precipitation in the reactions. The secondary metabolites tested were found to be present in different crude extracts with varied intensity and concentration showing positive reactions. The compounds which were present in abundance were symbolized as (+ + +), fairly present as (+ +) and slightly present as (+), whereas, negative reactions (−) represents the absence of those particular compounds in respective extracts. Some chemical test showed uncertain reaction which was symbolized as (±) is presented in Table 4.
Table 4.
Qualitative phytochemical screening of alcoholic crude herbal extracts
| Phytochemical | Tests | Observation | Butanolic | Ethanolic | Methanolic | |||
| Colda | Hotb | Colda | Hotb | Colda | Hotb | |||
| Alkaloids | Mayer's test | Precipitation / turbidity |
+ + | + + + | + | + + | + + | + + |
| Dragendorff's test | Precipitation / turbidity |
+ | + | + | + | + + | + | |
| Anthraquinones | Borntrager's test | Pink violet / red colour |
− | − | − | − | − | − |
| Cardiac | Legal test | Deep red colour | + | + | − | + | − | + |
| Glycosides | Keller-killiani test | Reddish brown ring |
− | − | − | ± | − | ± |
| Flavonoids | Alkaline reagent test |
Yellow color disappears |
− | + | − | + | + | + + |
| Shinoda test | Pink / magenta colour |
− | − | − | − | ± | − | |
| Saponins | Froth test | Frothing | − | − | − | − | − | − |
| Tannins | FeCl3 test | Blue-green precipitation |
− | − | − | − | − | − |
| Lead acetate test | Yellow precipitation |
− | − | − | − | + | + | |
| Terpenoids | Salkowski's test | Reddish violet color |
+ | + | + | + + | − | − |
a: Infusion (cold extraction), b: Soxhlet (hot percolation), +: positive (slightly present), ++: positive (fairly present), +++: positive (abundant), −: negative (absent), ±: doubtful reaction.
In this study, the alkaloids were detected with a high amount in all the extracts tested and the presence of precipitation / turbidity on the addition of Mayer's or Dragendorff's reagent confirmed the presence of alkaloids with highest intensity in hot extract of butanol. For cardiac glycosides, all the hot crude extracts and cold butanolic extract showed positive reactions with Legal test by forming deep red colour. Similarly, flavonoids reported its presence in all the hot crude extracts and cold methanolic extract by forming a yellow solution that turned colourless on the addition of HCl. However, a yellow precipitate which was formed on addition of lead acetate represents the presence of tannins only in the cold and hot methanolic extracts. In addition, the terpenoids were found to be present in hot and cold butanolic and ethanolic extracts as indicated by the appearance of reddish violet colour (Fig. 2). On the other hand, anthraquinones and saponins were not detected in all studied extracts as confirmed by the absence of pink-violet/ red colour and frothing, respectively.
Figure 2.

Showing results of preliminary phytochemical screening of crude herbal extract for Alkaloids -Dragendorff's test (Tube A) and Mayer's test (Tube B); Cardiac Glycosides - Legal test (Tube C); Flavonoids - Alkaline reagent test (Tube D); Tannins - Lead acetate test (Tube E) and Terpenoids - Salkowski's test (Tube F).
Phytochemical analysis studies of this plant have been reported earlier and different bioactive compounds have been identified. In a recent study, phytochemical analysis of different parts of P. juliflora extracts revealed distinct classes of secondary metabolites namely, tannins, phenolics, flavonoids, alkaloids, terpenes and steroids (Singh, 2012). In an another study the preliminary phytochemical screening of ethanolic leaf extract revealed the presence of tannins, phenolic acids, glycosides, flavonoids and alkaloids (Sathiya and Muthuchelian, 2008). Moreover, some compounds belonging to the alkaloid group of bioactive molecules were identified and characterized (Seetha Lakshmi et al., 2010). These include β-sitosterol (phytosterol), prosopidione (a terpenoid diketone) and three alkaloids namely secojuliprosopinal, 3'oxojuliprosopine and juliprosopine. Likewise, DART-MS analysis showed that P. juliflora leaves are a rich source of piperidine alkaloid and contain two diverse groups of alkaloids, one with an indolizidine ring in the centre of the molecule (viz., juliprosopine, juliprosine and juliprosinine) and other without indolizidine ring (viz., julifloridine, projuline and prosafrinine). Among them, juliprosopine and julifloridine were found to be present in high concentrations (Singh et al., 2011).
On the basis of results obtained from the preliminary antibacterial activity it was found that the soxhlet extraction gave a higher percentage yield of crude extracts while methanolic extract was considered to be the most active in respect to the wide range of inhibition zones against all test bacteria and presence of bioactive phytochemical compounds. Additionally, among all the crude extracts used for phytochemical screening, most of the chemical phytoconstituents were found to be present in an appreciable amount in the hot methanolic extract. Hence, hot extraction (soxhlet) procedure using methanol as a suitable solvent was selected for the mass extraction of crude phytoconstituents extract. The CES, after partitioning separately with Set-PCE and Set-HDB system, yielded six solvent and two aqueous fractions. The data revealed that the percentage yield of aqueous fractions was higher than the solvent fractions (Table 5). In Set-PCE, the highest percentage yield was obtained from AF-I (36.49%) followed by EF (1.16%), CF (0.75%) and PF (0.33%). Similarly, in Set-HDB, AF-II yielded highest value (32.92%) following BF (4.11%), DF (0.56%) and HF (0.03%).
Table 5.
Yield of solvent fractionation of CES obtained with two set of solvents
| Solvent Set | Fraction | Weight of empty | Weight of | Weight of | % yield |
| bottle (g) | bottle + fraction (g) | fraction (g) | |||
| Set-PCE | PF | 8.489 | 8.555 | 0.066 | 0.33 |
| CF | 8.421 | 8.570 | 0.149 | 0.75 | |
| EF | 8.494 | 8.725 | 0.231 | 1.16 | |
| AF-I | 8.350 | 15.647 | 7.297 | 36.49 | |
| Set-HDB | HF | 8.360 | 8.365 | 0.005 | 0.03 |
| DF | 8.485 | 8.597 | 0.112 | 0.56 | |
| BF | 8.462 | 9.284 | 0.822 | 4.11 | |
| AF-II | 8.472 | 15.055 | 6.583 | 32.92 |
Similar set of the solvent system were employed in our previous study on leaves of S. indicus in which highest percentage yield of aqueous fractions as compared to other solvent fractions were reported, where the aqueous fraction of Set-PCE, yielded highest value (14.63%) followed by chloroform (4.21%), ethyl acetate (3.07%) and petroleum spirit (1.31%) fractions and the aqueous fraction of Set-HDB yielded highest value (14.38%) followed by butanol (11.88%), dichloromethane (3.80%) and hexane (0.87%) fractions (Thakur et al., 2012).
Similarly, in an experiment conducted on the rhizomes of Drynaria quercifolia, the crude ethanolic extract (25.4 g) afforded petroleum ether (7.5 g), chloroform (7.8 g) and ethyl acetate (5.5 g) fractions (Khan et al., (2007). In an another study the crude methanolic extract (100.0 g) of aerial parts of Mitracarpus frigidus, partitioned successively to give hexane (22.0 g), dichloromethane (10.0 g), ethyl acetate (3.0 g), n-butanol (21.0 g) and the remaining hydromethanolic soluble fraction (39.0 g) (Fabri et al., (2009). Whereas, the crude ethanolic extract (104.9 g) of the leaves of Pera glabrata on partitioning yielded hexane, chloroform, ethyl acetate and water-methanol fractions representing 37.43 g, 10.14 g, 6.14 g and 24.03 g of the total extract, respectively (Cardoso-Lopes et al., 2009). In the same way, the n-hexane, chloroform and aqueous soluble fractions of a crude methanol extract (5.0 g) of the whole plant of Bryophyllum daigremontianum afforded 0.35 g, 0.15 g and 2.29 g materials, respectively (Nahar et al., 2008). Similarly, 5.0 g of methanol extract of the stem bark of Erythrina variegata afforded n-hexane (0.55 g), chloroform (1.50 g) and aqueous soluble materials (2.29 g) (Rahman et al., 2007). Likewise, in a study by the methanol extract (900.0 g) of the roots of Flemingia strobilifera was partitioned with dichloromethane and butanol to yield 18.0 g and 34.0 g of fractions, respectively (Madan et al., 2008).
The antibacterial activity of six solvents and two aqueous fractions at 25 mg/ml concentration followed a different trend as compared to preliminary antibacterial screening with the crude extracts. All fractions did not give a well-defined response against all ten bacteria while showing variable zones of inhibition. The results in terms of the diameter of the zone of inhibition of Set-PCE fractions prepared by the methanolic hot extraction method are illustrated in Table 6. The PF fraction was only effective against S. pyogenes giving a diameter of zone of inhibition of 14.67 ± 0.58 mm while the rest of the bacteria were not inhibited. The CF, EF and AF-I of Set-PCE were most active against S. epidermidis giving the diameter of zone of growth inhibition of 14.33 ± 1.15 mm, 11.00 ± 0.00 mm and 19.67 ± 0.58 mm, respectively (Fig 3, Plate 1, Well B, C, D). Against E. faecalis, both CF and EF showed the smallest inhibition zone of 6.00 ± 0.00 mm and 3.33 ± 0.58 mm, respectively (Fig 3, Plate 2, Well B, C). AF-I showed lowest activity with 4.00 ± 0.00 mm inhibition zone against S. typhimurium for which, the rest of the fractions were ineffective. However, none of the fractions could inhibit the growth of K. pneumoniae and S. aureus at concentration administered.
Table 6.
Diameter of zone of growth inhibition in mm of antibacterial activity of different fractions obtained with solvents of set-PCE
| Bacterial cultures | Solvent fraction (25 mg/ml) | |||
| PF | CF | EF | AF-I | |
| Bacillus subtilis | --------- | 10.00 ± 0.00 | 03.33 ± 0.58 | 13.00 ± 0.00 |
| Enterococcus faecalis | --------- | 06.00 ± 0.00 | 03.33 ± 0.58 | 09.67 ± 0.58 |
| Escherichia coli | --------- | 06.67 ± 0.58 | --------- | 12.33 ± 0.58 |
| Klebsiella pneumoniae | --------- | --------- | --------- | --------- |
| Pseudomonas aeruginosa | --------- | 08.67 ± 0.58 | 03.33 ± 0.58 | 12.00 ± 0.00 |
| Salmonella typhi | --------- | --------- | 05.00 ± 1.00 | 09.00 ± 0.00 |
| Salmonella typhimurium | --------- | --------- | --------- | 04.00 ± 0.00 |
| Staphylococcus aureus | --------- | --------- | --------- | --------- |
| Staphylococcus epidermidis | --------- | 14.33 ± 1.15 | 11.00 ± 0.00 | 19.67 ± 0.58 |
| Streptococcus pyogenes | 14.67 ± 0.58 | 13.33 ± 0.58 | 10.67 ± 0.58 | 14.67 ± 0.58 |
Antibacterial activity was expressed in terms of the diameter of zone of growth inhibition (mean ± S.D., n = 3)
Figure 3.
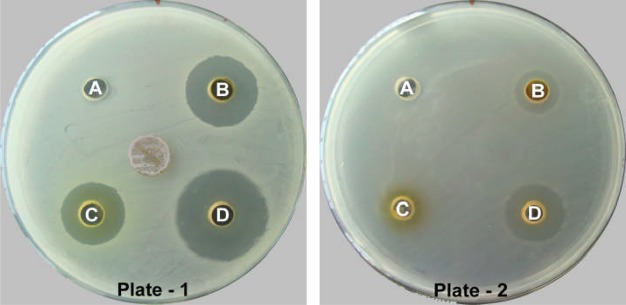
Showing antibacterial activity of solvent fractions of CES with set-PCE against Staphylococcus epidermidis (Plate-1) and Enterococcus faecalis (Plate-2).
In the same way, the results in terms of the diameter of the zone of inhibition of Set-HDB is illustrated in Table 7 in which HF, DF and AF-II fractions showed highest activity against S. pyogenes with 18.83 ± 0.76 mm, 16.33 ± 0.76 mm and 18.33 ± 0.58 mm zone of inhibition, respectively while BF showed maximum activity against P. aeruginosa with 22.67 ± 0.58 mm inhibition zone. The minimum inhibition zone of HF was observed against E. faecalis with 3.00 ± 0.00 mm, while that of DF against B. subtilis showing 5.00 ± 0.00 mm zone of inhibition. S. typhimurium was least affected by BF and AF-II with 4.33 ± 0.58 mm and 3.67 ± 0.58 mm zone of inhibition. However, none of the fractions could inhibit the growth of S. aureus at administered concentration. Of all the fractions, AF-I and AF-II were reported to be highly active in showing inhibitory activity against almost all the bacteria, but the most pronounced activity was shown against P. aeruginosa giving the largest zone of inhibition of 22.67 ± 0.58 mm (Fig 4, Plate 1, Well A) whereas least activity was shown by HF against E. faecalis giving the smallest zone of inhibition of 3.00 ± 0.00 mm (Fig 4, Plate 2, Well A).
Table 7.
Diameter of zone of growth inhibition in mm of antibacterial activity of different fractions obtained with solvents of set-HDB
| Bacterial cultures | Solvent fraction (25 mg/ml) | |||
| HF | DF | BF | AF-II | |
| Bacillus subtilis | 06.00 ± 0.00 | 05.00 ± 0.00 | 13.83 ± 0.29 | 13.67 ± 0.58 |
| Enterococcus faecalis | 03.00 ± 0.00 | 06.83 ± 0.29 | 12.67 ± 0.58 | 09.17 ± 0.29 |
| Escherichia coli | --------- | 06.50 ± 0.50 | 14.17 ± 1.04 | 13.33 ± 0.58 |
| Klebsiella pneumoniae | --------- | --------- | --------- | 04.83 ± 0.29 |
| Pseudomonas aeruginosa | --------- | 12.33 ± 0.58 | 22.67 ± 0.58 | 18.00 ± 0.00 |
| Salmonella typhi | --------- | 05.50 ± 0.50 | 13.00 ± 0.00 | 12.33 ± 0.58 |
| Salmonella typhimurium | --------- | --------- | 04.33 ± 0.58 | 03.67 ± 0.58 |
| Staphylococcus aureus | --------- | --------- | --------- | --------- |
| Staphylococcus epidermidis | --------- | 12.00 ± 1.00 | 18.33 ± 0.58 | 16.83 ± 0.29 |
| Streptococcus pyogenes | 18.83 ± 0.76 | 16.33 ± 0.76 | 18.33 ± 0.58 | 18.33 ± 0.58 |
Antibacterial activity was expressed in terms of the diameter of zone of growth inhibition (mean ± S.D., n = 3)
Figure 4.
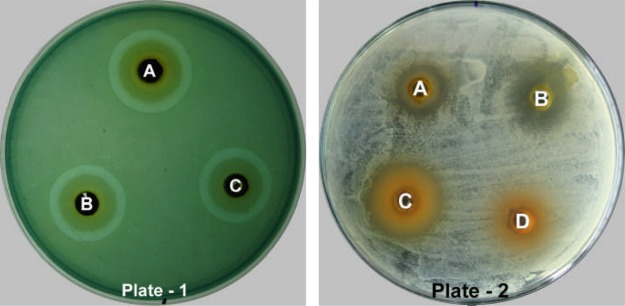
Showing antibacterial activity of solvent fractions of CES with set-HDB against Pseudomonas aeruginosa (Plate-1) and Enterococcus faecalis (Plate-2).
The data of our previous study revealed that the petroleum spirit, chloroform, hexane and dichloromethane fractions at 25 mg/ml concentration represented a broad-spectrum activity against five bacteria namely, B. subtilis, E. faecalis, S. epidermidis, S. typhi and S. pyogenes (Thakur et al., 2012). In another study, the dichloromethane fraction of Eucalyptus camaldulensis leaf extract at 10 mg/ml concentration exhibited antibacterial activity against B. subtilis (16 mm), Klebsiella spp. (15 mm), S. typhi (15 mm), S. aureus (13 mm) and P. aeruginosa (14 mm), but the petroleum ether fraction showed no activity on all test organisms similar in our study (Ayepola and Adeniyi, 2008). Similarly, petroleum ether fraction of Flabellaria paniculata leaf extracts (Abo et al., 2004) and Lumnitzera racemosa twigs (D'Souza et al., 2010) were also completely inactive against tested bacterial strains. Similar results were found in the chloroform soluble fraction of Acacia senegal which was inactive against K. pneumoniae and S. aureus in all three concentrations (1000 µg/ml, 3000 µg/ml, and 5000 µg/ml) whereas, the n-hexane soluble fraction showed dissimilar results by displaying activity against K. pneumoniae (Mudi and Salisu, 2009). Another investigation revealed that E. coli, S. aureus and K. pneumoniae were susceptible to the aqueous, butanolic and hexane fractions of the stem bark extract of Afzelia africana at a screening concentration of 10 mg/ml (Akinpelu et al., 2009).
In an another study the chloroform fraction of the leaf extracts of Flabellaria paniculata demonstrated antibacterial potential against S. aureus, P. aeruginosa, E. coli and K. pneumoniae (Abo et al., 2004) whereas, the n-butanol fraction obtained from twigs of Lumnitzera racemosa demonstrated good antibacterial activity at 50 µg/ml concentration against E. coli, K. pneumoniae, P. aeruginosa, S. typhi and S. aureus with a different degree of inhibition (D'Souza et al., 2010). In another study, the hexane fraction of Curcuma mangga showed 15.0 mm inhibition zone against P. aeruginosa, 9.5 mm against S. aureus and 13.5 mm against B. subtilis at concentration 500 mg/ml while the ethyl acetate fraction showed inhibition against P. aeruginosa, S. aureus and B. subtilis with inhibition zones 11.5, 9.0, 13.7 mm respectively at concentration 500 mg/ml (Philip et al., 2009). Similarly, the chloroform fraction (100 –400 mg/ml) of the leaves of Psidium guajava inhibited the growth of K. pneumoniae, whereas the ethyl acetate fraction showed significant inhibitory effects on the growth of S. aureus, S. typhi, K. pneumoniae and E. coli. The n-butanol fraction, at same the concentration, inhibited the growth of S. typhi and K. pneumoniae whereas, no effect was observed on S. aureus and E. coli (Geidam et al., 2007).
The data of our study revealed that, except PF, all the other organic fractions (CF, EF, HF, DF and BF) and aqueous fractions (AF-I and AF-II) represented a satisfactory response against tested bacterial strains, when administered 25 mg/ml concentration. The most sensitive bacteria was S. pyogenes showing sensitivity against all eight solvent fractions with considerable inhibition zones whereas S. aureus was highly resistance as no zones of inhibition was observed. The growth of this strain could not be inhibited by any of the extracts at concentration administered. It was also found that both the aqueous fractions consistently displayed better antibacterial activity as compared to fractions prepared in organic solvents. This result did not support the outcome of earlier studies carried out by Philip et al., (2009) and Geidam et al., (2007) in which the aqueous fractions of Curcuma mangga and Psidium guajava had no inhibitory effect on any bacteria used in their respective studies. On the other hand, the aqueous fraction of Pistacia integerrima and Aesculus indica were most active among all other fractions showing maximum inhibition zones of 19.66 mm and 16 mm, respectively, against B. subtilis (Bibi et al., 2011). The higher antibacterial activity of aqueous fractions may be due to the presence of polar compounds or could be attributed to the lack of solubility of active constituents in organic solvents. This explains the traditional practices of using water decoctions to cure various diseases (Agra et al., 2008).
Conclusion
The present study suggests that the crude extracts of Prosopis juliflora leaf powder and different solvent and aqueous fractions of its crude extracts possesses antibacterial potential against target bacterial type cultures. Qualitative phytochemical screening of crude extracts confirms that this plant is a rich source of active chemical constituents. The antibacterial activities of crude extracts and different fractions could be largely due to the cumulative effect of the phytochemicals present. Also, the absence of anthraquinones and saponins, in all the crude extracts implies that these compounds have no role in its overall antibacterial activity. The most active fraction can be further subjected to more detailed studies to identify the active principle(s) and its mechanism of action. Additional studies should emphasize the isolation and characterization of bioactive compounds and extensive in vitro and in vivo studies must be undertaken to substantiate the selection of active and nontoxic antibacterial phytoconstituents which could lead to the formulation of new antibacterial drugs. Furthermore, the present study may provide a scientific basis for the development of novel, safer and clinically effective medicine.
Acknowledgments
The authors are grateful to Shri S.N. Vijaywargia, Chancellor and Captain Ruchi Vijaywargia, Director CSRD, People's University, Bhopal, M.P., India for providing excellent research facilities and for their encouragement in carrying out this project. The authors are also thankful to Sarvajanik Jankalyan Parmarthik Niyas (SJPN), Bhopal, M.P., India for providing this research grant.
References
- 1.Abo KA, Olugbuyiro JAO. Phytochemical and antibacterial studies of extracts of Flabellaria paniculata. Afr J Biomed Res. 2004;7:35–36. [Google Scholar]
- 2.Agra MF, Silva KN, Basilio IJLD, Freitas PF, Barbosa-Filho JM. Survey of medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacogn. 2008;18(3):472–508. [Google Scholar]
- 3.Akinpelu DA, Aiyegoro AO, Okoh AI. Studies on the biocidal and cell membrane disruption potentials of stem bark extracts of Afzelia africana (Smith) Biol Res. 2009;42(3):339–349. [PubMed] [Google Scholar]
- 4.Ayepola OO, Adeniyi BA. The antibacterial activity of leaf extracts of Eucalyptus camaldulensis (Myrtaceae) J Appl Sci Res. 2008;4(11):1410–1413. [Google Scholar]
- 5.Bibi Y, Nisa S, Chaudhary FM, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complem Altern Med. 2011;11:52. doi: 10.1186/1472-6882-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso-Lopes EM, De Paula DMB, Barbo FE, De Souza A, Blatt CTT, Torres LMB, Young MOCM. Chemical composition, acetylcholinesterase inhibitory and antifungal activities of Pera glabrata (Schott) Baill. (Euphorbiaceae) Rev Bras Bot. 2009;32(4):819–825. [Google Scholar]
- 7.D'Souza L, Wahidulla S, Devi P. Antibacterial phenolics from the mangrove Lumnitzera racemosa. Indian J Mar Sci. 2010;39(2):294–298. [Google Scholar]
- 8.De S, Dey YN, Ghosh AK. Phytochemical investigation and chromatographic evaluation of the different extracts of tuber of Amorphophallus paeoniifolius (Araceae) Int J Pharm Biomed Res. 2010;1(5):150–157. [Google Scholar]
- 9.Fabri RL, Nogueira MS, Braga FG, Coimbra ES, Scio E. Mitracarpus frigidus aerial parts exhibited potent antimicrobial, antileishmanial and antioxidant effects. Bioresource Technol. 2009;100:428–433. doi: 10.1016/j.biortech.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Geidam YA, Ambali AG, Onyeyili PA. Phytochemical screening and antibacterial properties of organic solvent fractions of Psidium guajava aqueous leaf extracts. Int J Pharmacol. 2007;3(1):68–73. [Google Scholar]
- 11.Hari Prasad O, Aluru S, Kishore Kumar A, Navya A, Hari Krishna O, Bhaskar M, Papa Rao A, Reddy NR. Comparative evaluation of the antibacterial efficacy of P. juliflora and three commercially available mouthrinses: an in vitro study. J Pharm Res. 2011;4(7):2149–2151. [Google Scholar]
- 12.Harris PJOC, Pasiecznik NM, Smith SJ, Billington JM, Ramirez L. Differentiation of Prosopis juliflora (Sw.) DC. and P. pallida (H. & B. ex. Willd.) H.B.K. using foliar characters and ploidy. Forest Ecol Manag. 2003;180(1–3):153–164. [Google Scholar]
- 13.Hebbar SS, Harsha VH, Shripathi V, Hegde GR. Ethnomedicine of Dharwad district in Karnataka, India-plants used in oral health care. J Ethnopharmacol. 2004;94(2–3):261–266. doi: 10.1016/j.jep.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Haque E, Mukhlesur Rahman M, Mosaddik A, Rahman M, Sultana N. Isolation of antibacterial constituent from rhizome of Drynaria quercifolia and its sub-acute toxicological studies. DARU J Pharm Sci. 2007;15(4):205–211. [Google Scholar]
- 15.Madan S, Singh GN, Kumar Y, Kohli K, Singh RM, Mir SR, Ahmad S. A new flavanone from Flemingia strobilifera (Linn) R.Br. and its antimicrobial activity. Trop J Pharm Res. 2008;7(1):921–927. [Google Scholar]
- 16.Malik A, Kalidhar SB. A review of the chemistry and biological activity of Prosopis species. J Med Arom Plant Sci. 2005;27(4):675–705. [Google Scholar]
- 17.Mudi SY, Salisu A. Studies on brine shrimp lethality and activity of stem bark extract of Acacia senegal L. on respiratory tract pathogenic bacteria. Int J Biomed Health Sci. 2009;5(3):139–143. [Google Scholar]
- 18.Nahar K, Khan MGU, Rahman MS, Begum B, Rashid MA. Antimicrobial and cytotoxic activities of Bryophyllum daigremontianum. Dhaka Univ J Pharm Sci. 2008;7(1):99–101. [Google Scholar]
- 19.Napar AA, Bux H, Zia MA, Ahmad MZ, Iqbal A, Roomi S, Muhammad I, Shah SH. Antimicrobial and antioxidant activities of Mimosaceae plants; Acacia modesta Wall (Phulai), Prosopis cineraria (Linn.) and Prosopis juliflora (Swartz) J Med Plants Res. 2012;6(15):2962–2970. [Google Scholar]
- 20.Navya A, Hari Prasad O, Nanda Kumar Y, Sarma PVGK, Bhaskar M, Devi PUM. Antimicrobial, anti-proliferative activity and identification of drug candidates in phytochemicals of Prosopis juliflora. J Clin Cell Immunol. 2011 doi: 10.4172/2155-9899-10000S1. [DOI] [Google Scholar]
- 21.Pasiecznik NM, Felker P, Harris PJOC, Harsh LN, Cruz G, Tewari JOC, Cadoret K, Maldonado LJ. The Prosopis juliflora - Prosopis pallida complex: a monograph. Coventry, UK: HDRA; 2001. p. 172. [Google Scholar]
- 22.Patel JD, Kumar V. Annona squamosa L.: phytochemical analysis and antimicrobial screening. J Pharm Res. 2008;1(1):34–38. [Google Scholar]
- 23.Philip K, Malek SNA, Sani W, Shin SK, Kumar S, Lai HS, Serm LG, Rahman SNSA. Antimicrobial activity of some medicinal plants from Malaysia. Am J Appl Sci. 2009;6(8):1613–1617. [Google Scholar]
- 24.Prabha DS, Dahms HU, Malliga P. Assessment of acute and subacute oral toxicity of ethanolic extracts of Prosopis juliflora on Rattus norvegicus. J Herb Med Toxicol. 2012;6(1):61–65. [Google Scholar]
- 25.Rahman MZ, Sultana SJ, Faruquee CF, Ferdous F, Rahman MS, Islam MS, Rashid MA. Phytochemical and biological investigations of Erythrina variegata. Saudi Pharm J. 2007;15(2):140–145. [Google Scholar]
- 26.Reuben KD, Abdulrahman FI, Akan JOC, Usman H, Sodipo OA, Egwu GO. Phytochemical screening and in vitro antimicrobial investigation of the methanolic extract of Croton zambesicus Muell ARG. stem bark. Eur J Sci Res. 2008;23(1):134–140. [Google Scholar]
- 27.Sathiya M, Muthuchelian K. Investigation of phytochemical profile and antibacterial potential of ethanolic leaf extract of Prosopis juliflora DC. Ethnobot Leaflets. 2008;12:1240–1245. [Google Scholar]
- 28.Seetha Lakshmi B, Naidu KOC, Murthy YLN, Bobbarala V, Pandit N. Bio-efficacy of some medicinal plants against pathogens of cereal crops and phytochemical examination of Prosopis juliflora (Sw) Dc. J Pharm Res. 2010;3(2):356–360. [Google Scholar]
- 29.Senthilkumar N, Varma P, Gurusubramanian G. Larvicidal and adulticidal activities of some medicinal plants against the malarial vector, Anopheles stephensi (Liston) Parasitol Res. 2009;104:237–244. doi: 10.1007/s00436-008-1180-4. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh M, Malik AR, Meghavanshi MK, Mahmood I. Studies on some plant extracts for their antimicrobial potential against certain pathogenic microorganisms. Am J Plant Sci. 2012;3:209–213. [Google Scholar]
- 31.Singh S. Phytochemical analysis of different parts of Prosopis juliflora. Int J Curr Pharm Res. 2012;4(3):59–61. [Google Scholar]
- 32.Singh S, Swapnil, Verma SK. Antibacterial properties of alkaloid rich fractions obtained from various parts of Prosopis juliflora. Int J Pharma Sci Res. 2011;2(3):114–120. [Google Scholar]
- 33.Thakur R, Singh R, Jain N. Evaluation of antibacterial activity of Sphaeranthus indicus L. leaves. J Pharm Res. 2012;5(8):4382–4388. [Google Scholar]


