Abstract
Background
Flowers of Bauhinia kockiana were investigated for their anticancer properties.
Methods
Gallic acid (1), and methyl gallate (2), were isolated via bioassay-directed isolation, and they exhibited anticancer properties towards several cancer cell lines, examined using MTT cell viability assay. Pyrogallol (3) was examined against the same cancer cell lines to deduce the bioactive functional group of the phenolic compounds.
Results
The results showed that the phenolic compounds could exhibit moderate to weak cytotoxicity towards certain cell lines (GI50 30 – 86 µM), but were inactive towards DU145 prostate cancer cell (GI50 > 100 µM).
Conclusion
It was observed that pyrogallol moiety was one of the essential functional structures of the phenolic compounds in exhibiting anticancer activity. Also, the carboxyl group of compound 1 was also important in anticancer activity. Examination of the PC-3 cells treated with compound 1 using fluorescence microscopy showed that PC-3 cells were killed by apoptosis.
Keywords: Gallic acid, Bauhinia kockiana, pyrogallol, anticancer, apoptosis
Introduction
Cancer is largely characterized by uncontrolled proliferation and enlargements of abnormal cells within a tissue or an organ at all ages and in both sexes. These abnormal cells are capable of invading and destroying the neighbouring tissues and some have the ability to metastasize (Findler, 2003). Cancer can be treated in several ways, i.e. surgery, radiotherapy, immunotherapy and chemotherapy. Chemotherapy as a mode of treatment kills cancer cells by using anticancer drugs. Some anticancer drugs are derived from plants, such as taxol (found in Taxus brevifolia), combretastatin A-4 (from Combretum caffrum) (Srivastava et al., 2005), camptothecin (isolated from Camptotheca acuminate), vinblastine and vincristine (found in Catharanthus roseus) (Colegate & Molyneux, 2007).
Plants from the Leguminosae family have great economic importance as many of the plants are food plant, vegetation for livestock and folk medicines. Numerous plants from this family have medicinal properties and they are used in traditional medicine. For instance, soybeans are rich in isoflavone genistein which can suppress the growth of various cancer cells (Messina et al., 1994; Graham & Vance, 2003); leaf ash of Cassia abrus is applied to wounds and cuts (Jeeva et al., 2006); leaves of Crotolaria retusa are used to cure scabies and impetigo (Jeeva et al., 2006); and roots of Indigofera tinctoria are used as a remedy to counteract the effects of a poison (Jeeva et al., 2006).
Bauhinia kockiana Korth is cultivated as a garden ornamental plant in Malaysia. This plant is native to Peninsular Malaysia, but it can also be found in tropical forests in Thailand and Sumatra (Ong, 2006). It is also a medicinal plant with its several parts used to treat various health complications. For instance, its roots are used by the Kelabit ethnic group in Sarawak, eastern Malaysia to treat gonorrhoea, nervous debility, insomnia and fatigue (Fasihuddin et al., 1996; Ong, 2006). The infusion of bark and root are also used traditionally to treat toothache (Ong, 2006).
Evaluation of anticancer properties and isolation of anticancer agents in B. kockiana flower is of great interest in this study. Our preliminary investigation on the extracts showed that this plant exhibited moderately strong anticancer activity towards MCF-7 breast cancer cell. Bioassay-directed isolation of the ethyl acetate extract from flowers of B. kockiana is the main focus of this study. The purification of the ethyl acetate extract yielded two pure compounds and we report herein their cytotoxicity towards MCF-7 breast cancer cell, three prostate cancer cell lines (PC-3, LNCaP, DU145), and HCT-116 colon cancer cell. Morphological changes of the cancer cells treated with the active compound were studied and the cancer cell viability was determined. To the best of our knowledge, this is the first report on the phytochemicals isolated from B. kockiana using the bioassay-directed approach.
Material and Methods
General experimental procedures
Melting points were determined using a Kofhler hot stage apparatus equipped with a microscope XSP-12 model 500X and electro-thermal digital melting point apparatus. UV spectra were recorded on a U-1800 Hitachi spectrophotometer. IR spectra (KBr), were determined on a Perkin-Elmer FTIR SPECTRUM BX spectrophotometer (in wavenumber, cm−1). 1H-NMR and 13C-NMR spectra were acquired using a JOEL FTNMR (400 MHz), spectrometer, using tetramethylsilane (TMS), as an internal standard (chemical shift values were quoted in ppm, δ). Gas chromatography - mass spectrometry (GC-MS), was recorded on a Shimadzu GCMS-QP5050A spectrometer with 5% phenyl methylsiloxane capillary column of dimension 30.0 m × 250 µm x 0.25 µm, and the carrier gas used was helium gas at 1 mL/min. The GC oven used was a Shimadzu GC-17A which was programmed from 80 – 325 °C at a rate of 10 °C min−1, with an initial hold time of 1 min and final hold time of 10 min, while the MS was operated at 70 eV. Analytical chromatographic analyses were performed using a liquid chromatography Agilent Technologies series 1200, which consisted G1311A quaternary pump, G1315B diode array detector, G1322A vacuum degasser and equipped with a reversed-phase column, Eclipse XDB-C18 (250 × 4.6 mm), with 5 µm particle size. 5% and 40% v/v acetonitrile in water were used as the mobile phase. Solvent gradient was performed as follows: 5% to 40% acetonitrile in a linear gradient, from 0 to 60 min, and the flow rate was fixed at 1 mL/min. Column chromatography was performed using silica gel (Merck Kiesegel PF254 7749 silica gel for vacuum column chromatography and Merck Kieselgel PF254 9385 silica gel, 230 – 400 mesh for gravity column chromatography), and Sepahadex LH-20 (Sigma Aldrich). Analytical thin layer chromatography (TLC), was carried out using commercially available Merck TLC aluminium sheets pre-coated with Kieselgel 60 F254 (40 mm × 80 mm × 0.2 mm).
Chemicals
RPMI 1640 medium, penicillin-streptomycin and trypsin EDTA (1X), were purchased from GIBCO (Invitrogen Corporation, CA, USA). Fetal bovine serum (FBS), phosphate-buffered saline (PBS), MTT (3-[4,5-dimethylthiazol-2yl]-2, 5-diphenyltetrazolium bromide), acridine orange (AO), and pyrogallol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO), deuterated acetone and propidium iodide (PI) was purchased from Fluka (Switzerland). Doxorubicin was purchased from Ebewe (Austria), and paclitaxel was obtained from Laboratory filaxis (Argentina). The solvent for HPLC analysis was of HPLC grade while all other organic solvents used were of analytical grade, obtained from Fisher Chemicals (Springfield, NJ). The water used was of Milipore quality.
Plant materials
B. kockiana flowers were collected from Klang Valley in Peninsular Malaysia and were identified by Mr. Anthonysamy Savarimuthu, a plant taxonomist formerly of Universiti Putra Malaysia (UPM). Voucher specimen of B. kockiana (MUM-LEGUM-001), was deposited in the herbarium of School of Science, Monash University Sunway Campus, Malaysia.
Cell culture
MCF-7, PC-3, LNCaP, DU145, and HCT-116 cancer cell lines were purchased from The American Type Culture Collection (ATCC). All cancer cells were grown in RPMI-1640 media supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin and the cells were incubated at 37 °C in an atmosphere of 5% CO2 and 95% air to 60 – 70% confluence.
Extraction and isolation
Fresh flowers of B. kockiana (1.7 kg), were collected, washed with distilled water, and freeze-dried using a freeze dryer. The dried flowers (550 g), were powdered and percolated sequentially with 12 L of solvents in the following order: hexane, dichloromethane, ethyl acetate, n-butanol, methanol and water. The plant material was soaked for 48 hours in each solvent and the extraction was repeated for several times with fresh solvent until successive extraction was achieved. The hexane, dichloromethane, ethyl acetate and methanol extracts were dried individually using a rotary evaporator at 40 °C, the n- butanol extract was concentrated at 55 °C, and the water extract was dried using a freeze-dryer. 30 g of the ethyl acetate extract was fractionated with vacuum liquid chromatography over Merck 7749 silica gel with chloroform - ethyl acetate - methanol (in increasing polarity), as eluants. The polarity of solvents was increased to yield four major fractions, labeled FE1 - FE4. All fractions were subjected to cytotoxicity screening. A part of fraction FE2 (15.0 g), was separated using Merck 9385 silica gel and eluted in a gradient manner with chloroform - ethyl acetate - methanol, in increasing polarity to yield five fractions (FE2-1 – FE2-5), and these fractions were subjected to cytotoxicity screening. Fraction FE2-3 (1.5 g), was further purified in a similar manner using a silica gel column using Merck 9385 silica gel with stepwise gradient elution with chloroform - methanol and yielded 1 (40 mg), and 5 other fractions (FE2-3-1 – FE2-3-5). Fraction FE2-3-4 (0.7 g) was further purified using Sepahadex LH-20 and eluted with methanol to give rise to 2 (52 mg). The purities of 1 and 2 were confirmed using RP-HPLC; the molecular masses were obtained using GCMS and their chemical structures characterized using NMR and FTIR.
Phytochemical analysis of sequential extracts: flavonoid and tannin
Analyses for flavonoids and tannins were determined as described by Parekh and Chanda (2009). 0.1 g of extract was dissolved in 1.0 mL distilled water (dH2O), and filtered. For screening of flavonoids, 10 mg magnesium ribbon was added into the filtrate, followed by the addition of 0.05 mL concentrated sulphuric acid. The presence of a magenta red colour within three minutes confirmed the presence of flavonoids. For the detection of tannins, a few drops of ferric chloride (0.01 g/mL) were added into the filtrate. The presence of tannin is confirmed by appearance of blue-black precipitates.
Cytotoxicity assay: MTT cell viability assay
The assay was performed as described by Wong et al. (2011). Cancer cells were plated in 96-well flat bottom tissue culture plates at initial seeding densities approximately 3000 cells per well in 180 µL of RPMI-1640 media. The cells were then incubated for 16 hours at 37 °C in an atmosphere of 5% CO2 and 95% air to allow the cell attachment onto the wells. 20 µL of extracts or phytochemicals (ranging from 0.1 µg/mL to 100 µg/mL), were added into the respective wells (four replicates for each concentration), and the plates were incubated at 37 °C, 5% CO2 and 95% air for 96 hours (untreated cells were served as control), before 50 µL of MTT (2 mg/mL), was added into each well. The plates were again incubated at 37 °C in an atmosphere of 5% CO2 and 95% air for 4 hours to allow metabolism of MTT by cellular mitochondrial dehydrogenase. The supernatant was aspirated and 100 µL of DMSO was added into each well to dissolve the formazan crystals formed, and the absorbance of formazan was measured at 550 nm using microplate reader. The semi-log dose-response curve (percentage growth vs. concentrations) was plotted, and three dose-response parameters (GI50, TGI and LC50) were determined.
Fluorescent microscopy of PC-3 cells under AO/PI double staining
Morphological changes and cell death of PC-3 prostate cancer cells were observed using AO/PI double staining. 30000 PC-3 prostate cancer cells in 900 µL of RPMI-1640 media per well were plated in 24-well flat bottom tissue culture plates. Cells were incubated for 16 hours at 37 °C in an atmosphere of 5% CO2 and 95% air to allow the attachment onto the wells. 100 µL of compound 1 (ranging from 1.76 µM to 58.8 µM), were added into the respective wells (100 µL of culture medium were added into the untreated cells as control). The cells were incubated at 37 °C, 5% CO2 and 95% air for 96 hours. The media of each well were then aspirated and were collected into sterile micro-centrifuge tubes, while 1 mL of trypsin-EDTA was added into each well for 5 mins., to detach the cells from the plates. All cells were transferred into the sterile tubes and were centrifuged at 12000 g for 5 mins. The supernatants were discarded and the pelleted cells were washed and re-suspended in 100 µL ice-cold PBS, followed by re-centrifugation to remove the remaining media. Supernatants were discarded and 20 µL of fluorescent dyes (containing 5µg/mL AO and 5µg/mL of PI in PBS) was added into the cell pellet and cells were re-suspended. 20 µL of the freshly stained cell suspension was mounted onto a glass slide and observed under UV-fluorescence microscope excited at blue light (488 nm) and the images of AO/PI stained cells were captured.
Statistical analysis
All measurements were carried out in triplicate. Statistical analyses were performed using a one-way analysis of variance ANOVA, and the significance of the difference between means was determined by Duncan's multiple range tests. Differences at P < 0.05 were considered statistically significant. The results were presented as mean values ± SD (standard deviations).
Results and Discussion
Cytotoxicity of extracts towards MCF-7 breast cancer cell
Cytotoxicity assay was used throughout the bioassay-directed isolation procedure, and MCF-7 breast cancer cell line was selected for assessment. Screening showed that ethyl acetate, methanol and n-butanol extracts displayed cytotoxicity. The ethyl acetate extract is more cytotoxic than methanol and n-butanol extract, with lower GI50, TGI and LC50 (26.0 ± 1.8 µg/mL, 38.9 ± 2.2 µg/mL, 51.8 ± 4.8 µg/mL, respectively). This study shows that the bioactive compounds have medium polar properties and phytochemical tests showed that they could be tannins and/or flavonoids. The hexane, dichloromethane and water extracts were found to be inactive (GI50 > 100 µg/mL) (Table 1).
Table 1.
Cytotoxicity towards MCF-7 breast cancer cell of flower extracts of B. kockiana
| Extracts | Cytotoxic activity (µg/mL) | Phytochemical screening |
||
| GI50 | TGI | LC50 | ||
| Hexane | >100 | >100 | >100 | n.t. |
| Dichloromethane | >100 | >100 | >100 | n.t. |
| Ethyl acetate | 26.0 ± 1.8a | 38.9 ± 2.2a | 51.8 ± 4.8a | Tannin |
| Methanol | 31.5 ± 2.6b | 49.0 ± 6.1b | 66.6 ± 9.5b | Tannin, flavonoid |
| n-Butanol | 58.6 ± 1.3c | >100 | >100 | n.t. |
| Water | >100 | >100 | >100 | n.t. |
Note: GI50 = concentration that inhibits growth by 50%; TGI = concentration that inhibits growth by 100%; LC50 = concentration that kills 50% of treated cells. Data are expressed as means ± SD obtained from 3 independent experiments. “n.t.” represents not tested. For each column, values followed by the same letter (a–b) are statistically insignificant (P> 0.05) as determined by ANOVA.
Structural elucidation of compounds 1 and 2
The chemical structure of the components isolated from B. kockiana flower was accomplished by comparing mass spectrum, UV spectrum, IR, 1H-NMR and 13C-NMR data with literature.
Compound 1 isolated from Fraction FE2-3 was identified as garlic acid (IUPAC name is 3,4,5-trihydroxybenzoic acid; molecular formula C7H6O5). It was isolated as yellow needles which melted at 250 – 252 °C. It appeared as one spot with Rf value 0.77 on TLC in acetone:ethyl acetate (6:4), solvent system, and the spot turned ferric chloride solution to dark blue, indicating the presence of phenol group. Mass spectrometry showed that it has a molecular ion peak of m/z 170 (100%), and other major fragments were seen at 153 (95%), and 125 (20%). This was in agreement with the fragmentation pattern of an aromatic carboxylic acid. The fragmentation pathway of an aromatic carboxylic acid is the loss of OH to form C6H5O3CO+ (m/z = 153), followed by loss of CO to form C6H5O3 (m/z = 125). The absorption spectrum of garlic acid exhibits two peaks in the UV range, at 230 and 270 nm. The structure was further substantiated by the IR spectrum, where a broad peak at 3000 – 3400 cm−1 (O-H, H-bonded), sharp peaks at 1690 cm−1 (C=O stretch), 1600–1680 cm−1 (aromatic C=C stretch) and 1260 cm−1 (C-O stretch) were observed. 1H-NMR spectrum of gallic acid showed only one signal at δH 7.15 (2H, s, H-2, H-6) while 13C-NMR spectrum showed five signals: δC 145.9 (C-3, C-5), δC 138.6 (C-4), δC 121.9 (C-1), δC 110.0 (C-2, C-6), and δC 167.8 for carbonyl group. The spectra data are in agreement with that for gallic acid reported by Chen et al. (2008).
Compound 2 isolated from FE2-3-4 was identified as methyl gallate (IUPAC name is methyl 3,4,5-trihydroxybenzoate; molecular formula C8H8O5). It was isolated as white crystal, with melting point 199 – 201°C. It appeared as one spot on the TLC plate with Rf value 0.77 developed using acetone:methanol:chloroform (4:2:4) solvent system. The spot turned to dark blue when sprayed with ferric chloride reagent, showing the presence of phenol group. Mass spectrometry showed that methyl gallate has a molecular ion peak of m/z 184 (80%), and other major fragments at 153 (100%) and 125 (95%). It is compatible to the fragmentation pathway of an alkyl benzoate ester, where the the alkoxy group is first lost to form C6H5O3CO+ (m/z = 153), followed by loss of CO to form C6H5O3 (m/z = 125). Two maximum absorption peaks were present in the UV spectrum, at 218 and 274 nm. The chemical structure was also supported by the IR spectrum, with the absorption bands appeared at 3480 cm−1 (O-H stretch, intermolecular H-bond), 1700 cm−1 (C=O stretch) and 1600–1680 cm−1 (aromatic C=C stretch). The 1H-NMR spectrum showed two major signals: a methoxyl signal at δH 3.77, and aromatic protons signal at δH 7.10 (2H, s, H-2, H-6) while the 13C-NMR spectrum showed six signals: δC 146.0 (C-3, C-5), δC 138.7 (C-4), δC 121.6 (C-1), δC 109.7 (C-2, C-6), δC 167.1 for carbonyl group and δC 51.8 for methoxy group. The spectra data obtained are in agreement with that of methyl gallate reported by Nishioka et al. (1998), and Fecka and Cisowski (2005).
Cytotoxicity of compounds 1 and 2 towards various cancer cell lines
This was the first to report that compounds 1 and 2 were isolated from B. kockiana and the cytotoxicity of these two phenolic compounds was evaluated using various cancer cell lines. Cytotoxicity of these compounds was examined on MCF-7 breast cancer cell, and also on various cancer cell lines, namely prostate cancer cells (PC-3, LNCaP, and DU145), and HCT-116 colon cancer cell.
Both phenolic compounds displayed selective toxicity towards the cancer cell lines (Table 2). Compound 1 exhibited moderate cytotoxicity towards MCF-7 and PC-3 cells; weak cytotoxicity towards LNCaP and HCT-116 cells; and not active against DU145 cells. However, compound 2 exhibited weak cytotoxicity towards MCF-7 and PC-3 cells, and were not active against three other cell lines (LNCaP, DU145 and HCT-116 cells) (Table 2). It was noticed that compound 1 is more cytotoxic than 2. Exception was DU145 cell, where both compounds were equally inactive (GI50 > 100 µM). A higher dose to achieve 50% growth inhibition may be required. Positive controls, doxorubicin and paclitaxel exhibited very strong cytotoxic effect towards the cancer cells tested, except that doxorubicin was not active towards HCT-116 cells.
Table 2.
Cytotoxicity of phytochemicals on various cancer cell lines
| Compound | Cytotoxicity (µM) |
Cancer cell lines | ||||
| MCF-7a | PC-3a | LNCaPc | DU145b | HCT-116c | ||
| 1 | GI50 TGI LC50 |
29.6 ± 3.4 61.5 ± 0.8 93.5 ± 1.8 |
22.8 ± 2.0 45.8 ± 3.5 68.9 ± 5.0 |
75.0 ± 3.5 > 100 > 100 |
> 100 > 100 > 100 |
71.3 ± 2.8 > 100 > 100 |
| 2 | GI50 TGI LC50 |
86.0 ± 2.5 > 100 > 100 |
75.4 ± 5.6 > 100 > 100 |
> 100 > 100 > 100 |
> 100 > 100 > 100 |
> 100 > 100 > 100 |
| 3 | GI50 TGI LC50 |
36.8 ± 1.8 76.9 ± 1.2 > 100 |
28.6 ± 1.7 60.0 ± 5.3 91.4 ± 9.0 |
35.3 ± 4.5 68.9 ± 6.6 > 100 |
8.45 ± 0.96 18.7 ± 0.36 28.9 ± 0.24 |
39.0 ± 1.1 72.5 ± 5.0 > 100 |
| Doxorubicin | GI50 TGI LC50 |
0.450 1.90 5.50 |
0.700 4.00 20.0 |
1.50 4.00 10.0 |
1.20 4.00 15.0 |
>100 >100 >100 |
| Paclitaxel | GI50 TGI LC50 |
0.018 0.060 4.00 |
0.010 0.070 >100 |
0.035 0.080 >100 |
0.030 0.100 >100 |
0.200 20.0 >100 |
Note: GI50 = concentration that inhibits growth by 50%; TGI = concentration that inhibits growth by 100%; LC50 = concentration that kills 50% of treated cells. Data are expressed as means ± SD obtained from 3 independent experiments. a Statistically significant (P < 0.05) between compound 1, 2 and 3 in term of GI50 TGI and LC50. b Compound 1 and 2 were inactive. c Compound 1 and 3 were statistically significant (P < 0.05), in terms of GI50 and TGI, compound 2 was inactive. Cytotoxic effect was categorized into strong (GI50 < 10 µM), moderate (GI50 10 – 50 µM), weak (GI50 51 – 100 µM) and non cytotoxic/inactive (GI50 >100 µM).
Studies had reported that pyrogallol moiety of these two compounds could exhibit cytotoxic effect against cancer cells (Inoue et al., 1995; Fernandes et al., 2010). To further substantiate that pyrogallol moiety was one of the important functional structures of the phenolic compounds in exhibiting biological activity on various cancer cells, pyrogallol (3), a decarboxylated structure of gallic acid was also tested on the five cancer cell lines. This structure-based study would provide useful information in future drug design, molecular modification, and determining the potency which could be useful in proposing effective therapeutic agents and understanding their interaction with the biological targets.
As seen in Table 2, pyrogallol demonstrated strong to moderate activity. It was recently discovered that the number and position of hydroxyl groups of pyrogallol moiety were equally important for the phenolic compounds to exhibit anti-proliferate activity. It was reported that the cancer cell growth inhibition is related to the number of hydroxyl groups in the phenolic ring, in both flavonoid and non-flavonoid compounds. Fernandes et al. (2010) has evaluated the cancer cell growth of four flavonoids with similar structural features. The results showed that flavonoids which carry trihydroxyl group at ring B were more potent than dihydroxyl compounds of similar structures. In addition, non-flavonoid or phenolic compounds which consisted of trihydroxyl groups were also more cytotoxic than those which have only dihydroxyl groups, i.e. pyrogallol was more cytotoxic than catecol, and gallic acid has stronger inhibition activity than protocatechuic acid (Fernandes et al., 2010). Inoue et al. (1995), also found that deletion or methylation of the hydroxyl group would result in complete loss of cytoxicity. The position of three hydroxyl groups was found to be related to the anti-proliferate activity of cancer cells. Phloroglucinol that carries three hydroxyl groups at C-1, C-3 and C-5 of benzene ring was less active than pyrogallol, where the three hydroxyl groups were positioned at C-1, C-2 and C-3 of benzene ring (Fernandes et al., 2010).
It is interesting to report that the carboxyl group of gallic acid could demonstrate stronger antiproliferative activity towards selected cancer cells. Gallic acid displayed stronger inhibition activity against MCF-7 and PC-3 cells than pyrogallol. This suggests the carboxyl group of gallic acid may have contributed to the growth inhibition but the mechanism is still unknown. However, the inhibitory effect of carboxyl group was not seen in LNCaP, DU145 and HCT-116 cells.
Inoue et al. (1995) reported that the three phenolic hydroxyl groups are important in exhibiting cytotoxicity, but the carboxyl group was not involved in the cell inhibition activity. However, our study found that the presence of carboxyl group in 1 is equally important in exhibiting the inhibition activity. Compounds 1 and 2 differ in the functional group attached to C-1 position of the aromatic ring. Compound 1 has a carboxyl group at C-1 while compound 2 has carbomethoxy group attached to C-1. Isuzugawa et al. (2001) found that replacement of carboxyl group of 1 with carbomethoxy group (as in 2) could lead to remarkably decreased or complete loss of activity. This explains the results obtained (Table 2), where 2 exhibited weaker inhibition activity than 1 in all cell lines tested. Similar observations were also reported in other compounds in the literatures. Zouhiri et al. (2000) found that the existence of carboxyl group at C-7 of styrylquinolines is important in biological activity and the replacement with carbomethoxy group at C-7 position would cause reduction or complete loss of activity. Lee et al. (1988), also discovered that esterification of carboxyl group at C-17 of ursolic acid would lead to decrease in toxicity. Hata et al. (2002) reported that the carboxylic group of betulinic acid at C-17 position is important for the cancer cell apoptosis, where the existence of carboxylic group enhances its potency. As a conclusion, the structural requirements of 1 for biological activity are three hydroxyl groups at C-3, C-4 and C-5 position of pyrogallol moiety and carboxyl group at C-1 position.
Apoptotic death of PC-3 cells induced by compound 1
Compound 1 exhibited the strongest growth inhibition activity towards PC-3 prostate cancer cell. PC-3 cell was established from human prostatic adenocarcinoma metastatic to bone and it has higher metastatic potential compared to DU145 (moderate) and LNCaP (low), cell lines (Pulukuri et al., 2005). Our study found that the growth of PC-3 cells was not affected at low concentration of compound 1 treatment. Gradual reduction in cell growth was noticed as the concentration of 1 increase, where GI50 and TGI were achieved at 22.8 µM and 45.8 µM. The cell death of prostate cancer induced by 1 is dose dependent, but not time dependent (Veluri et al., 2006).
A cell could be killed by two death-induced pathways: apoptosis and necrosis. Apoptosis is a mode of cell death which occurs in the body under normal physiological conditions (Ishaque & Al-Rubeai, 2004), where the process was regulated and controlled. It was important in removing abnormal cells, regulating balance in tissue mass, in embryogenesis, immune system regulation and also in cancer chemotherapy treatment (Ishaque & Al-Rubeai, 2004). Cells may undergo necrosis when they are exposed to extreme physiological situation, such as infection, trauma and overdose of cytotoxic drug, which may result in damage to the plasma membrane (Kerr et al., 1994; Ishaque & Al-Rubeai, 2004).
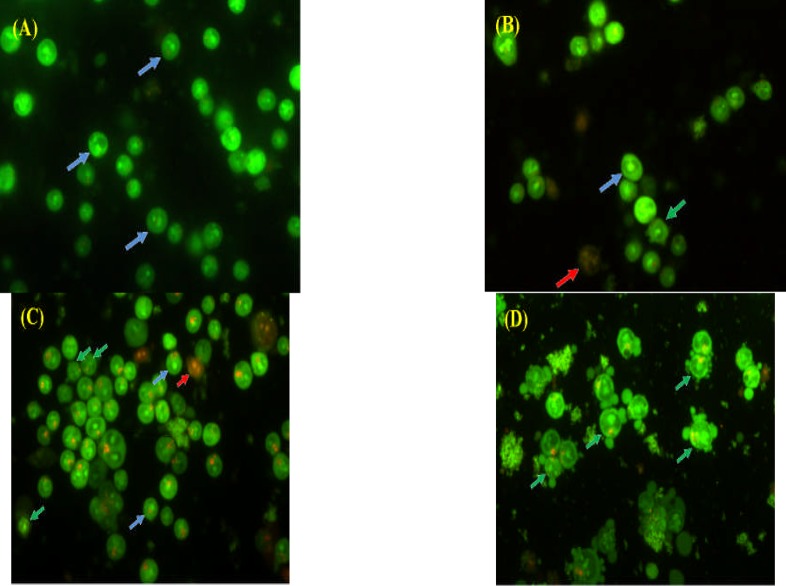
Apoptotic and necrotic cells differ in morphology (Fig. 2) and biochemical features and can lead to different physiological significance to the organism. To determine the mode of cell death induced by compound 1 in this study, the treated PC-3 cells were stained with AO/PI double stains and examined. One of the advantages of using AO/PI double stain was that they show specificity in viable, apoptotic, late apoptotic and necrotic cells. AO can diffuse into living cells and bound double stranded DNA, with preference over AT-rich regions of normal nucleic acid while PI is only permeable into late apoptotic and necrotic cell, and it would intercalate every 4 – 5 base pairs of nucleic acid without any sequence preference (Foglieni et al., 2001). AO bound to the DNA of viable cells would show green emission while the late apoptotic and necrotic cells which could only uptake the PI stain will be seen as red colour cells when the stained cells are exposed to fluorescence blue light. At the lowest treatment concentration (1.76 µM), viable cells were appeared as green with normal cell shape and regular nuclear structure (Fig 3A). As the treatment concentration increased, blebbing on the intact external cell surface and nuclear margination (Fig 3B – D), indicated that the cells have undergone apoptosis.
Figure 2.
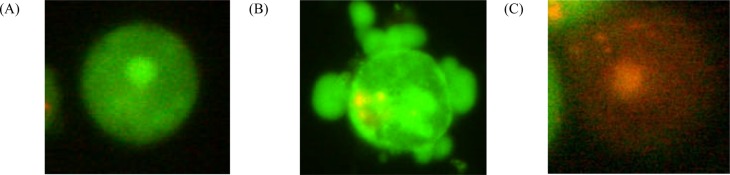
Fluorescence micrographs of PC-3 prostate cancer cells after AO/PI double staining.
(A) Viable cells with green fluorescence and with normal structure, (B) Apoptotic cell is green in colour with blebbing and nuclear margination, (C) Necrotic cell with red fluorescence and intact nuclei membrane (400X).
Figure 3.
Fluorescence micrographs of compound 1-treated PC-3 prostate cancer cells at (A) 1.76 µM, (B) 5.88 µM, (C) 17.6 µM, and (D) 58.8 µM at 96 hours with AO/PI double staining. Arrows indicating viable cells (blue), apoptotic cells (green), and necrotic cells (red) (400X).
Compound 1 is well known to be a good antioxidant and displayed anticancer activities in in vitro and in vivo studies (Saleem et al., 2002). Studies reported that it has higher sensitivity to kill cancer cells, compared to normal cells (Inoue et al., 1995; Sakaguchi et al., 1998). Inoue et al (1995), and Sakaguchi et al. (1998) reported that fragmentation of DNA, condensation of chromatin, formation of apoptotic bodies were seen in compound 1 treated cancer cells which indicated that the cells were killed via apoptotic pathway. It was reported that the apoptosis in cancer cells was induced by intracellular Ca2+ and reactive oxygen species (ROS) (such as H2O2, O2•-), and mitochondrial dysfunction (You & Park, 2010). Recently, compound 1 was found to increase the intracellular ROS level in cancer cells and elevates the level of Ca2+ and H2O2 to induce apoptosis in various cancer cells (You & Park, 2010). This finding was supported by earlier study by Sakaguchi et al. (1998), where no cancer cell apoptosis and DNA fragmentation were noticed when intracellular Ca2+ chelator (BAPTA-AM), calmodulin inhibitor (W-7) and radical scavengers (ascorbic acid, catalase, superoxide dismutase and thiol antioxidant n-acetyl-L-cysteine), were added into the culture.
Veluri et al. (2006), found that 12 hours exposure of 25 µg/mL (equivalent to 147 µM), of compound 1 to DU145 cells had resulted in activation of caspase-9 and caspase-3, and also generated poly (ADP-ribose) polymerase (PARP), a major substrate of activated caspase-3. In addition, 24 hours of compound 1 exposure could also induce early apoptosis on DU145 cells (Agarwal et al., 2006; Veluri et al., 2006). It promotes apoptosis by activating the pro-apoptotic protein and inactivating the anti apoptotic protein (Chen et al., 2009), and this has led to proteolytic cleavage of PARP, caspase-3 and caspase-9 (Agarwal et al., 2006; Veluri et al., 2006; Chen et al., 2009). This showed that DU145 cells have undergone intrinsic apoptotic pathway, induced by compound 1. Besides, application of high dose of 1 (approximately 294 µM) could cause massive apoptotic cell death (Veluri et al., 2006).
Studies discovered that compound 1-treated DU145 cells were arrested in both S and G2-M phases in cell cycle progression (Agarwal et al., 2006; Chen et al., 2009), while the number of sub-G1 cells remained unchanged (You & Park, 2010). Kinases Chk1 and Chk2 were activated, and cyclin B1, Cdc25C and Cdc2 were down-regulated (Chen et al., 2009). Therefore cell cycle was arrested and apoptosis was induced.
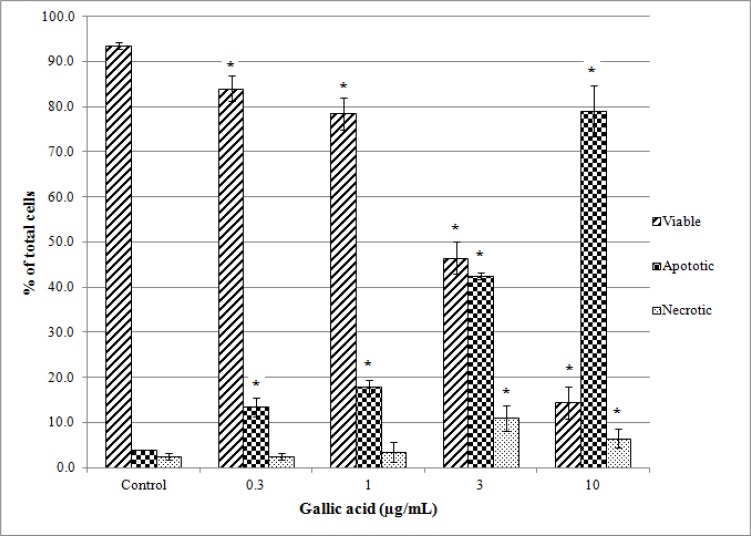
Figure 4 showed that PC-3 cells treated with compound 1 have undergone apoptosis in dose-dependent manner: the number of viable cells decreased significantly while the number of apoptotic cells increased with concentration of 1. Only small number of necrotic cells (approximately 10%) was noticed. This showed that 1 is PC-3 cells apoptotic inducer and it kills the cells via programmed cell death. Moderate potency of 1 in suppressing prostate cancer cell growth showed that it can be used as an alternative cancer chemo-preventive agent, as it is capable of killing cancer cells without triggering acute inflammatory response. It could also work synergistically with doxorubicin in suppressing the growth of DU145 prostate cancer cells (Chen et al., 2009) and this suggests that combination of 1 with clinical chemotherapeutic agents may reduce the dose of anticancer drugs delivered in cancer treatment.
Figure 4.
Induction of apoptosis and necrosis by compound 1 in PC-3 prostate cancer cells at various concentrations. Results are expressed as means ± SD obtained from 3 independent experiments. In each experiment, three treatment replications were carried out. * Significant differences compared with control cells with P < 0.05.
Figure 1.
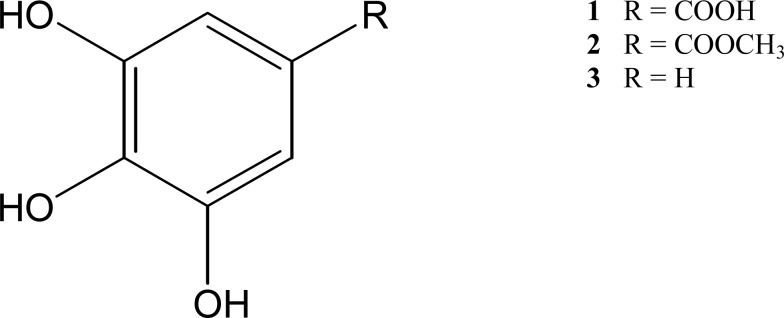
Chemical structure of compounds 1–3
Acknowledgements
The authors are thankful to Monash University Sunway Campus for financial support. We are grateful to Mr. Anthonysamy Savarimuthu (formerly of UPM) for identifying the plant.
List of non-standard abbreviations
- AO
Acridine orange
- ATCC
The American Type Culture Collection
- dH2O
Distilled water
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- GC-MS
Gas chromatography - mass spectrometry
- GI50
Concentration that inhibits growth by 50%
- LC50
Concentration that kills 50% of treated cells
- MTT
3-[4,5-dimethylthiazol-2yl]-2, 5-diphenyltetrazolium bromide
- PARP
Poly (ADP-ribose) polymerase
- PBS
Phosphate-buffered saline
- PI
Propidium iodide
- ROS
Reactive oxygen species
- TGI
Concentration that inhibits growth by 100%
- TLC
Thin layer chromatography
- TMS
Tetramethylsilane
- UPM
Universiti Putra Malaysia
References
- 1.Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25c-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol cancer therapeutics. 2006;5:3294. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 2.Chen HM, Wu YOC, Chang FR, Hsu HK, Hsieh YOC, Chen COC, Yuan SS. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Liu YM, Yang S, Song BA, Xu GF, Bhadury PS, Jin LH, Hu DY, Liu F, Xue W, andZhou X. Studies on the chemical constituents and anticancer activity of Saxifragaa stolonifera (L) Meeb. Bioorg Med Chem. 2008;16:1337–1344. doi: 10.1016/j.bmc.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 4.Colegate SM, Molyneux RJ. Bioactive Natural Products: Detection, Isolation and Structural Determination. Florida: CRC Press; 2007. [Google Scholar]
- 5.Fasihuddin BA, Ipor IB, Din LB. Medicinal plants used by the Kelabit community in Bario, Sarawak. In: Ghazally I, Murtedza M, Din LB, editors. Chemical prospecting in the Malaysian forest. Petaling Jaya: Pelanduk Publication; 1996. 43–46 pp. [Google Scholar]
- 6.Fecka I, Cisowski W. Tannins and flavonoids from the Erodium cicutarium Herb. Zeitschrift fr Naturforschung B. 2005;60b:555–560. [Google Scholar]
- 7.Fernandes I, Faria A, Azevedo J, Soares S, Calhau C, Freitas VD, Mateus N. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J Agri Food Chem. 2010;58:3785–3792. doi: 10.1021/jf903714z. [DOI] [PubMed] [Google Scholar]
- 8.Findler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 9.Foglieni C, Meoni C, Davalli AM. Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol. 2001;115:223–229. doi: 10.1007/s004180100249. [DOI] [PubMed] [Google Scholar]
- 10.Graham PH, Vance CP. Legumes: Importance and constraints to greater use. Plant Physiol. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata K, Hori K, Takahashi S. Differentiation-and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J Nat Prod. 2002;65(5):645–648. doi: 10.1021/np0104673. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Suzuki R, Sakaguchi N, Li Z, Takeda T, Ogihara Y, Jiang BY, Chen Y. Selective inductive of cell death in cancer cells by gallic acid. Biol Pharm Bull. 1995;18(11):1526–1530. doi: 10.1248/bpb.18.1526. [DOI] [PubMed] [Google Scholar]
- 13.Ishaque A, Al-Rubeai M. Monitoring apoptosis. In: Al-Rubeai M, editor. Fussenegger, Cell Engineering. Vol. 4. Netherlands: Kluwer Academic Publishers; 2004. 281–306 pp. [Google Scholar]
- 14.Isuzugawa K, Ogihara Y, Inoue M. Different generation of inhibitors against gallic acid-induced apoptosis produces different sensitivity to gallic acid. Biol Pharrm Bull. 2001;24(3):249–253. doi: 10.1248/bpb.24.249. [DOI] [PubMed] [Google Scholar]
- 15.Jeeva S, Kiruba S, Mishra BP, Venugopal N, Dhas SSM, Regim GS, Kingston C, Kavitha A, Sukumaran S, Raj ADS, Laloo ROC. Weeds of Kanyakumari district and their value in rural life. Ind J Traditional Knowledge. 2006;5(4):501–509. [Google Scholar]
- 16.Kerr JFR, Winterford CM, Harmon BV. Apoptosis. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Lin YM, Wu TS, Zhang DOC, Yamagishi T, Hayashi T, Hall IH, Chang JJ, Wu RY, Yang TH. The cytotoxic principles of Prunella vulgaris, Psychotria serpens, and Hyptis capitata: Ursolic acid and related derivatives. Planta Med. 1988;54(4):308–311. doi: 10.1055/s-2006-962441. [DOI] [PubMed] [Google Scholar]
- 18.Messina MJ, Persky V, Setchell KDR, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutri, and Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 19.Nishioka T, Kawabata J, Aoyama Y. Baicalein, an α-glucosidase inhibitor from Scutellaria baicalensis. J Nat Prod. 1998;61:1413–1415. doi: 10.1021/np980163p. [DOI] [PubMed] [Google Scholar]
- 20.Ong HOC. Tanaman hiasan: Khasiat makanan & ubatan. Bhd, Selangor: Utusan Publications & Distributors Sdn.; 2006. [Google Scholar]
- 21.Parekh J, Chanda S. In vitro antifungal activity of methanol extracts of some Indian medicinal plants against pathogenic yeast and moulds. African J Biotech. 2008;7(23):4349–4353. [Google Scholar]
- 22.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA Interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. The J Biol Chem. 2005;280(43):36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sakaguchi N, Inoue M, Ogihara Y. Reactive oxygen species and Intracellular Ca2+, common signals for apoptosis induced by gallic acid. Biochem Pharmacol. 1998;55:1973–1981. doi: 10.1016/s0006-2952(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 24.Saleem A, Husheem M, Härkonen P, Pihlaja K. Inhibition of cancer growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmaco. 2002;81:327–336. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 25.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogen. 2006;27(7):1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 26.Wong HOC, Sagineedu SR, Lajis NH, Loke SOC, Stanslas J. Andrographolide induces cell cycle arrest and apoptosis in PC-3 prostate cancer cells. African J Pharm Pharmacol. 2011;5(2):225–233. [Google Scholar]
- 27.You BR, Park WH. Gallic acid-induced lung cancer is related to glutathione depletion as well as reactive oxygen species increase. Toxicol in vitro. 2010;24:1356–1362. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Zouhiri F, Mouscadet JF, Mekouar K, Desmaële D, Savouré D, Leh H, Subra F, Bret M, Auclair C, d'Angelo J. Structure-activity relationships and binding mode of styrylquinolines as potent inhibitors of HIV-1 integrase and replication of HIV in cell culture. J Med Chem. 2000;43(8):1533–1540. doi: 10.1021/jm990467o. [DOI] [PubMed] [Google Scholar]