Abstract
Background
Increased nitric oxide (NO), neuronal inflammation and apoptosis have been proposed to be involved in excitotoxicity plays a part in many neurodegenerative diseases. To understand the neuro-protective effects of propolis, activities of Nitric oxide synthase (NOS) and caspase-3 along with NO and tumor necrosis factor-α (TNF-α) levels were studied in cerebral cortex (CC), cerebellum (CB) and brain stem (BS) in rats supplemented with propolis prior to excitotoxic injury with kainic acid (KA).
Materials and methods
Male Sprague-Dawley rats were divided into four groups (n=6 rats per group) as Control, KA, Propolis and KA+Propolis. The control group and KA group have received vehicle and saline. Propolis group and propolis + KA group were orally administered with propolis (150mg/kg body weight), five times every 12 hours. KA group and propolis +KA group were injected subcutaneously with kainic acid (15mg/kg body weight) and were sacrificed after 2 hrs. CC, CB and BS were separated, homogenized and used for estimation of NOS, caspase-3, NO and TNF-α by commercial kits. Results were analyzed by one way ANOVA, reported as mean + SD (n=6 rats), and p<0.05 was considered statistically significant.
Results
The concentration of NO, TNF-α, NOS and caspase-3 activity were increased significantly (p<0.001) in all the three brain regions tested in KA group compared to the control. Propolis supplementation significantly (p<0.001) prevented the increase in NOS, NO, TNF-α and caspase-3 due to KA.
Conclusion
Results of this study clearly demonstrated that the propolis supplementation attenuated the NOS, caspase-3 activities, NO, and TNF-α concentration and in KA mediated excitotoxicity. Hence propolis can be a possible potential protective agent against excitotoxicity and neurodegenerative disorders.
Keywords: Nitric oxide, TNF-α, Caspase-3, Excitotoxicity, Propolis, Rat Brain
Introduction
The exact mechanisms involved in onset and progression of neurodegenerative diseases are still poorly defined, but excitotoxicity is recognized as one of the mechanisms involved (Atlante et al., 2001; Mattson, 203). Excessive neuronal excitation involving the excitatory glutamate receptors is recognized as an important underlying mechanism in neurodegenerative disorders. However, the exact mechanism of how excitotoxicity is implicated in neurodegeneration still needs further investigation. Kainic acid (KA) induced status epilepticus was associated with both apoptotic and necrotic cell death and induction of heat sensitive proteins in hippocampus and cortical regions of rodent brain (Akbar et al., 2001; Kato et al., 1999; White, 2002). The exact mechanisms contributing to increased concentration of nitric oxide (NO) in excitotoxicity are not well established. Earlier studies reported that nitric oxide synthase (NOS) knockout mice were more severely affected by epileptic activity than the controls and that the response to NO during epilepsy depends on its concentration (Itoh and Watanabe, 2009). It was also indicated that NO may be regarded as an anticonvulsant and proconvulsant substance in relation to convulsions induced by pentylenetetrazole (PTZ) (Itoh and Watanabe, 2009).
Excitotoxicity is commonly induced in experimental animals by KA, a 30-fold more potent glutamate agonist (Sperk, 1994). Effects of KA are mediated via activation of the kainite receptors that respond to the neurotransmitter glutamate and include the induction of inflammatory responses, production of cytokines and neuronal death (Ullah et al., 2014). The molecular mechanisms by which KA induces excitotoxicity and cell death remain unclear; however, oxidative stress and the activation of proinflammatory cytokines are major contributors (Wong et al., 2005). The cytokines and other inflammatory molecules secreted by activated glia cells can modify the outcome of disease progression. Thus, antioxidant and anti-inflammatory treatment could attenuate or prevent KA-induced neurodegeneration (Zhang and Zhu, 2011). Additionally, KA increases neuronal excitability, production of reactive oxygen species (ROS), and lipid peroxidation (Hasegawa et al 2008; Bruce and Baudry, 1995; Sun et al., 1992). Microglial activation and astrocytes proliferation are the other characteristics of KA-induced neurodegeneration. Both in vitro and in vivo studies demonstrate that KA induces cell death via accumulation of intracellular calcium, which stimulates ROS production and mitochondrial dysfunction, thereby leading to neuronal cell death (Wong et al., 2005; Hilton et al., 2005). There is evidence for activation of calpain-and caspases-induced neural apoptosis following KA exposure (Smialowska et al., 2011). Besides oxidative stress and intracellular calcium overload, KA can activate molecular mechanisms leading to depletion of neuronal energy stores and thereby activating the alternative cell death pathways (Choi, 1987; Culmsee et al., 2001; McCullough, 2005).
Honey bee propolis has been widely used as a folk medicine and proposed to be protective on neurodegenerative disorders (Ha et al., 2010; Kwon et al., 2004). It has been shown to have broad biological activities, which are principally attributed to the presence of flavonoids (Isla et al., 2001) and caffeic acid phenyl ester (CAPE) (Natarajan et al., 1996). The prevailing opinion is that the broad biological activities of flavonoids and CAPE are related, in part, to their anti-inflammatory and anti oxidant actions (Isla et al., 2001; Natarajan et al., 1996). Recent studies shown that propolis supplementation reduced the oxidative stress and nitric oxide levels in KA-mediated exicitotoxicity (Swamy et al., 2014). Therefore the present study was conducted to assess the neuroprotective effects of the bee product propolis , by estimating the concentration of TNF-α, and NO, along with activities of NOS and caspase-3 in cerebral cortex (CC), cerebellum (CB) and brain stem (BS) of rats supplemented with propolis and subjected to KA mediated excitotoxicity.
Material and Methods
Propolis collection and ethanol extraction
Honey bee propolis was obtained from Muzmi Resources, Kota Bharu, Malaysia. It was subjected to 80% ethanol extract as per the procedure of Isla et al. (2001) as described by Swamy et al. (2014). Single lot of propolis was used for extraction and used for administration to rats.
Animals
Male Sprague Dawley rats weighing 200 – 250 grams were used for the study. The animals had free access to food and water. They were fed with commercial feed and had access to water ad libitum. They were housed under standard condition of constant temperature; humidity and a 12h light/dark cycle were maintained. Animal handling and experimental design was approved by the Animal ethics committee of Universiti Sains Malaysia, Health campus, Kubang Kerian, Malaysia [USM / Animal Ethics Approval / 20011 / (68) (296)].
Experimental Study
The rats were divided in to one control group and three study groups; KA group, propolis group and propolis + KA group with six rats in each group. Control group and KA group received vehicle and saline. Propolis group and propolis + KA were orally administered with ethanol-extracted propolis (150mg/kg body weight), five times every 12 hours as described by Kwon et al. (2004). KA group and propolis + KA group rats were given subcutaneous injection of kainic acid (15mg/kg body weight) (Milatovic et al., 2002) and were sacrificed after 2hrs of KA injection. Control group and propolis group rats were given normal saline and sacrificed after 2hrs of saline injection. After the rats sacrificed by decapitation the brain regions −CC, CB, and BS were separated according to the procedure described by Sadasivudu and Lajtha (1970). Each of the brain regions was weighed and used for the preparation of homogenates in 0.05M phosphate buffer pH 7.3.
Enzyme assay
Nitric oxide synthase
NOS activity was estimated by the method of Yui et al. (1991) as described by Swamy et al. (2011a), in which the stable end products, NOx, were estimated using the Nitric Oxide Synthase assay Kit from Calbiochem, U.S.A. (Catalogue Number 482702) The optical density was measured at 540 nm using VersaMax ELISA, Microplate Reader. NOS activity was expressed as nano mole NOx /g wet tissue/ hour Caspase-3: Caspase-3 activity was assayed using Caspase-3/CPP32 colorimetric assay kit from BioVision Research Products, Milpitas, California, USA. The optical density was measured at 400 nm using VersaMax ELISA, Microplate Reader Caspase activity was expressed as nano mole pNA/g wet tissue/hour
Estimations of NO, and TNF-α
NO was estimated as NOx (Nitrate/Nitrite) by Griess reaction after conversion of nitrate to nitrite by nitrate reductase, as described by Swamy et al (2011a) using the commercially available Nitric Oxide Assay Kit from Cayman Chemical Company (Catalogue number 780001; Ann Arbor, Michigan, USA). The optical density was measured at 540 nm using VersaMax ELISA, Microplate Reader. TNF-α was estimated using ELISA kit from Abnova GmbH, Geramny (Catalog No KA0280). The optical density was measured at 620 nm using VersaMax ELISA, Microplate Reader
Statistical analysis
Results were reported as mean + standard deviation (SD) from 6 rats for each parameter studied. Statistical analysis of results was done by one-way analysis of variance (ANOVA) followed by post hoc analysis using Bonferroni's test, using the SPSS software (version 20) to determine the statistical significance of difference in values between the control and study groups. p value of < 0.05 was taken as statistically significant at 95% confidence interval.
Results
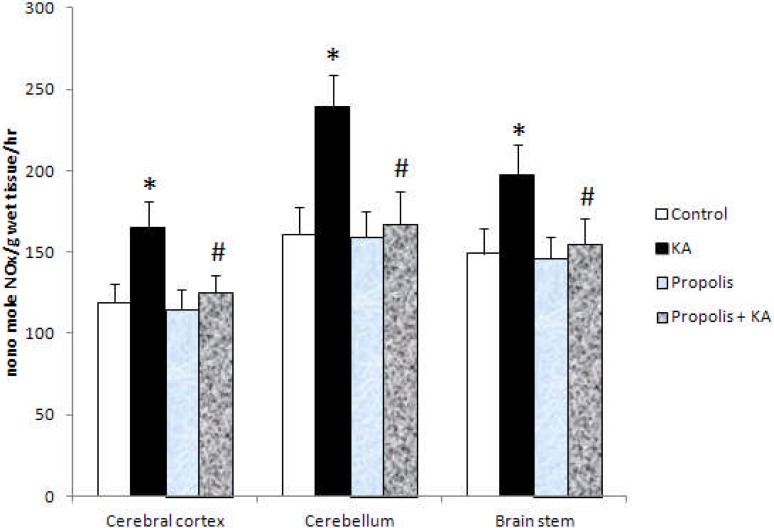
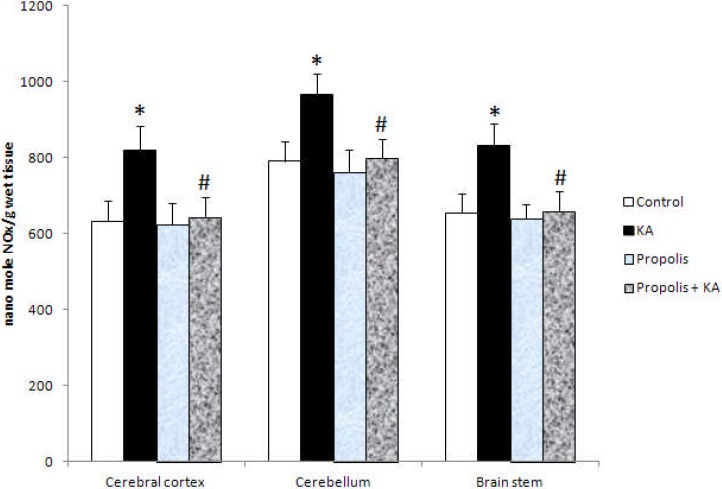
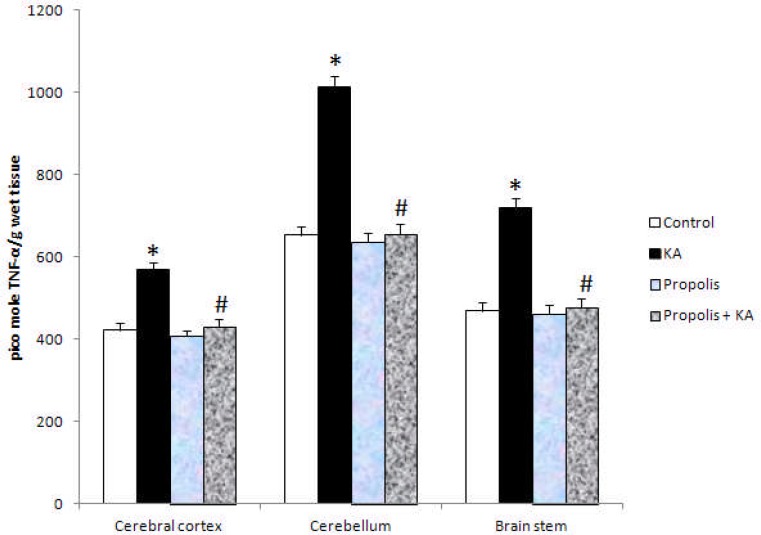
The activity of NOS and NO concentration were increased significantly (p<0.001) in all the three brain regions tested in KA group compared to control group, but the increased activity of NOS and NO concentration by KA were prevented by prior supplementation of propolis (Figure 1 and Figure 2). There were no significant differences in the activity of NOS and NO concentration between control and propolis as well as propolis + KA group (Figure 1 and Figure 2). The concentration of TNF-α was increased significantly (p<0.001) in all the three brain regions tested in KA group compared to control group, but the increase of TBARS concentration by KA was prevented (p<0.001) by prior supplementation with propolis (propolis + KA group) (Figure 3). There was no significant difference in TNF-α concentration between control and propolis as well as propolis + KA group (Figure 3).
Figure 1.
The activity of NOS in KA mediated excitotoxicity and propolis supplementation Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
Figure 2.
The Concentration of NOx in KA mediated excitotoxicity and propolis supplementation Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
Figure 3.
The concentration of TNF-α in KA mediated excitotoxicity and propolis supplementation Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
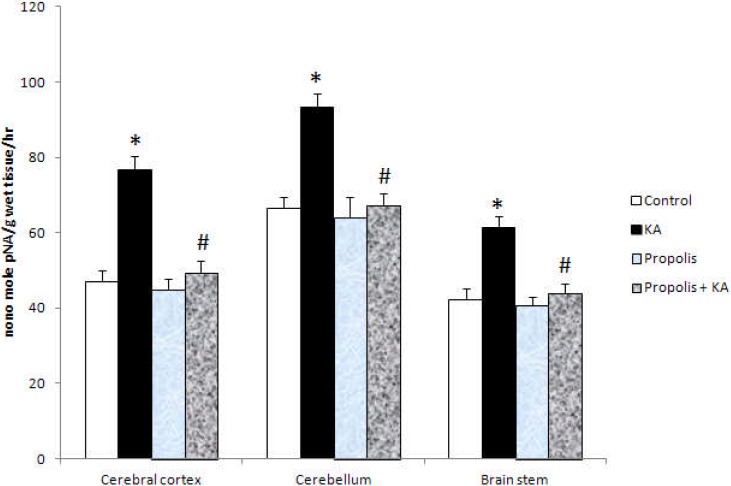
The activity of caspase-3 was increased significantly (p<0.001) in KA group compared to control and propolis + KA group and the increased activity of caspase by KA was prevented (p<0.001) by supplementation of propolis (propolis + KA group) (figure 4). There were no significant differences in caspase activity between control and propolis as well as propolis + KA group (Figure 4).
Figure 4.
The activity of caspase-3 in KA mediated excitotoxicity and propolis supplementation Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
Discussion
Excitotoxicity is the pathological process where neurons are damaged or killed by overstimulation of glutamate receptors in the Central Nervous System (CNS) by excitatory amino acids and it is considered to be contributing factor in the pathogenesis of various neurodegenerative disorders in the CNS (Wang, et al., 2005; Zhang and Zhu, 2011, Zheng, et al., 2011). It was earlier reported that stimulation of glutamate-KA receptors induces neuronal NO release, which in turn modulates glutamate transmission (Alabadi et al., 1999; Nakaki etal., 2000). NO induces changes in neuronal and signaling-related functions by several ways (Prast and Philippu, 2001). Glutamate excitotoxicity contributes to a variety of disorders in the central nervous system, which is triggered primarily by excessive Ca2+ influx arising from overstimulation of glutamate receptors, followed by disintegration of the endoplasmic reticulum (ER) membrane and ER stress, the generation and detoxification of reactive oxygen species as well as mitochondrial dysfunction, leading to neuronal apoptosis and necrosis (Schinder, et al., 1996; Nicholls, 2004). The literature findings implicate neuronal NO generation in the pathogenesis of both direct and secondary excitotoxic neuronal injuries in vivo. Although NMDA receptors likely contribute critically to neuronal injury in various acute conditions, several observations support the hypothesis that AMPA/KA receptors may be of greater importance to the neurodegenerative process (Carriedo et al., 1998, 2000). The production of NO represents one of the principle features of activated macrophage/microglia, and NO is a major effector in the innate immunity (Stuehr, et al., 1991). Earlier work indicated that KA excitotoxicity was associated with energy depletion and oxidative stress (Silva-adaya, et al., 2008). AMP-activated protein kinase (AMPK), the multifunctional metabolic and energy sensor in the brain, is activated by conditions of cellular energy depletion (Kemp, et al., 1999; Ramamurthy and Ronnett, 2006). It was reported that KA induced activation of AMPK is associated with induction of apoptotic cell death in immortalized mouse hippocampal cells (HT22) and in primary cultures of prenatal rat hippocampal neurons (Ullah, et al., 2011). Korean black bean anthocyanins, naturally occurring antioxidants, were attenuated the neurotoxicity induced by KA in vitro (Ullah, et al., 2011). Their study reported that neuroprotective effects against KA-induced excitotoxicity were attributed to attenuation of numerous KA-induced processes such as ROS accumulation, increase in AMPK activation, perturbation of Ca2+ homeostasis, loss of mitochondrial integrity, inhibition of Bax accumulation, inhibition of the release of mitochondrial cytochrome-c into the cytoplasm, and reduction in cellar content of activated caspase-3(Ullah, et al., 2011).
Honey bee propolis has been used to maintain health. Pharmacological activities such as anticancer, anti inflammatory, antibiotic, ant oxidative, antifungal, anesthetic and cytostatic have been ascribed to ethanolic extracts of propolis (Isla et al., 2001). Propolis has been shown to have broad biological activities, which are principally attributed to the presence of flavonoids (major component; rutin, quercetin, galangin, etc.), phenolic compounds and CAPE (Isla et al., 2001). The beneficial actions of propolis contents namely flavonoids, phenolic compounds and CAPE are related, in part, to their anti-inflammatory and anti oxidant actions (Isla et al., 2001; Kwon et al., 2004). Propolis contents namely phenolic compounds and CAPE were shown to cross blood brain barrier (Medic-Saric et al., 2009; Silva et al., 2013). CAPE was shown to protects against the loss of dopaminergic neurons induced by 6-OHDA in rats (Silva et al., 2013).
Our earlier studies have shown that NOS and NO were increased along with increased oxidative stress markers in KA mediated excitotoxicity and epilepsy rat models (Swamy, et al., 2009, 2011a). We have also reported the amelioration of oxidative stress and NO concentration by propolis suplimentation in KA mediated excitotoxicity (Swamy, et al., 2014). Considering that oxidative stress is central to KA induced excitotoxic damage, anti-oxidant and anti-inflammatory treatments may attenuate or prevent the KA mediated neurodegeneration (Zhang and Zhu, 2011). It has been reported that anti-inflammatory substances lucidone (Senthil Kumar et al., 2010), curcumin (Jung et al., 2006), and phenantroindolizdine alkaloids reduce NO production observed in inflammation (Yang et al., 2006). The results of this study showed the prevention of increased NOS activity and NO concentration is in favor of our earlier report (Swamy, et al., 2014). The suppression of proinflammatory marker TNF-α concentration by propolis supplementation in KA mediated excitotoxicity suggests that propolis can protect the neuronal damage by KA. The increased activity of Caspase-3 seen in this study was also prevented by propolis supplementation. Hence the beneficial effects of propolis may be due to its antioxidant, anti-inflammatory and anti-αpoptotic properties.
Conclusion
The results of this study showed that propolis supplementation has prevented the increased NOS and caspase-3 activities along with NO and TNF-α concentration in KA mediated excitotoxicity in rat brain. Thus propolis can be a possible potential protective agent against excitotoxicity and neurodegenerative disorders.
Acknowledgement
This study received support from Universiti Sains Malaysia-Research University grant (A/C No: 1001/PPSP/813052). The findings of the study were presented in the International Conference on Natural Products 2014, 18th – 19th March 2014 at Palm Garden Hotel, Putrajaya, Malaysia.
References
- 1.Akbar MT, Wells DJ, Latchman DS, de Belleroche J. Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glail and neuronal cells compared to heat shock protein 70 and caspase-3 following kainite administration. Brain Res Mol Brain Res. 2001;93(2):148–163. doi: 10.1016/s0169-328x(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 2.Alabadi J, Thibault JL, Pinard E, Seylaz J, Lasbennes F. 7-Nitroindazole a selective inhibitor of nNOS increases hippocampal extracellular glutamate concentration in status epilepticus induced by kainic acid in rats. Brain Res. 1999;839(2):305–312. doi: 10.1016/s0006-8993(99)01749-7. [DOI] [PubMed] [Google Scholar]
- 3.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497(1):1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 4.Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic Biol Med. 1995;18(6):993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- 5.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. Rapid Ca2+ entry through Ca2+ permeable AMPa/kainite channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci. 1998;1(19):7727–7738. doi: 10.1523/JNEUROSCI.18-19-07727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca2+overload and ROS generation in spinal motor neurons in vitro. J Neuroscience. 2000;20(1):240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMPK-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17(1):45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 9.Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010;485(3):143–147. doi: 10.1016/j.neulet.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa T, Takano F, Takata T, Niiyama M, Ohta T. Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globules. Phytochemistry. 2008;6(3):747–753. doi: 10.1016/j.phytochem.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol modulation of kainic acid-induced calcium elevation in neonatal hippocampal neurons. Endocrinology. 2006;147(3):1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- 12.Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001;76(2):165–170. doi: 10.1016/s0378-8741(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Watanabe M. Paradoxical facilitation of pentylenetetrazole-induced convulsion susceptibility in mice lacking neuronal nitric oxide synthase. Neuroscience. 2009;159(2):735–743. doi: 10.1016/j.neuroscience.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 14.Jung KK, Lee HS, Cho JY, Shin WOC, Rhee MH, Kim TG, Kang JH, Kim SH, Hong S, Kang SY. Inhibitory effect of curcumin on nitric oxide production from lipopolisaccharide-activated primary microglia. Life Sci. 2006;79(21):2022–2031. doi: 10.1016/j.lfs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Katoh-Semba R, Takeuchi IK, Ito H, Kamei K. Responses of heat shock proteins hsp27, alphaB-crystalline, and hsp70 in rat brain after kainic acid-induced seizure activity. J Neurochem. 1999;73(1):229–236. doi: 10.1046/j.1471-4159.1999.0730229.x. [DOI] [PubMed] [Google Scholar]
- 16.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Writters LA. Dealing with energy demand: The AMPK-activated protein kinase. Trends Biochem Sci. 1999;2(1):22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YS, Park DH, Shin EJ, Kwon MS, Ko KH, Kim WK, Jhoo JH, Joo WK, Wie MB, Jung BD, Kim HOC. Antioxident propolis attenuates Kainate-induced neurotoxicity Vai adenosine A1 receptor modulation in the rat. Neurosci Lett. 2004;355(3):231–235. doi: 10.1016/j.neulet.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 18.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMPK-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280(21):20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 19.Mattson MP. Excitotoxic and excitoprotective mechanism: abundant targets for the prevention and treatment of neurodegenerative disorders. NeuroMol Med. 2003;3(2):65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 20.Medic-Saric M, Rastija V, Bojic M, Males Z. From functional food to medicinal product: systematic approach in analysis of polyphenolics from propolis and wine. Nutr J. 2009;8:33. doi: 10.1186/1475-2891-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milatovic D, Gupta ROC, Dettbarn WD. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002;957(2):330–337. doi: 10.1016/s0006-8993(02)03669-7. [DOI] [PubMed] [Google Scholar]
- 22.Nakaki T, Mishima A, Suzuki E, Shintani F, Fujii T. Glufosinate ammonium stimulates nitric oxide production through N-methyl-D-aspartate receptors in rat cerebellum. Neurosci Lett. 2000;290(3):209–212. doi: 10.1016/s0304-3940(00)01363-x. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K, Singh S, Burke Jr TR, Grunberg D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kB. Proc Natl Acad Sci USA. 1996;93(17):9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4(2):1491–77. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 25.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64(1):51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Ramamurthy S, Ronnett GV. AMPK-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574(1):85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadasivudu B, Lajtha A. Metabolism of amino acids in incubated slices of mouse brain. J Neurochem. 1970;17(8):1299–1311. doi: 10.1111/j.1471-4159.1970.tb03379.x. [DOI] [PubMed] [Google Scholar]
- 28.Schinder AF, Olson EOC, Spitzer NOC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16(19):6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senthil Kumar KJ, Hsieh HW, Wang SY. Anti-inflammatory effect of lucidone in mice via inhibition of NF-kappB/MPK kinase pathway. Int Immunopharmacol. 2010;10(4):385–392. doi: 10.1016/j.intimp.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Silva-αdaya D, Perez-De La Cruz V, Herrera-Mundo MN, Mendoza-Maccedo K, Villeda-Hernandez J, Bininda Z, Ali SF, Santamria A. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: antioxidant and neuroprotective effects of L-carnitine. J Neurochem. 2008;105(3):677–689. doi: 10.1111/j.1471-4159.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- 31.Silva R B, Santos N D G, Martins N M, Ferreira D S A, Barbosa, et al. Caffeic acid phenyl ester protects against the dopaminergic neuronal loss induced by 6-Hydroxydopamine in rats. Neuroscience. 2013;233:86–94. doi: 10.1016/j.neuroscience.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Smialowska M, Golembiowska K, Kajta M, Zieba B, Dziubina A, Domin H. Selective mGluR1 antagonist EMQMCM inhibits the kainite-induced excitotoxicity in primary neuronal cultures and in the rat hippocampus. Neurotox Res. 2011;21(4):379–392. doi: 10.1007/s12640-011-9293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42(1):1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 34.Stuehr D J, Cho H J, Kwon N S, Weise M F, Nathan C F. Purification and characterization of the cytokineinduced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. PNS, UA. 1991;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun AY, Cheng Y, Bu Q, Oldfield F. The biochemical mechanisms of the excitotoxicity of kainic acid Free radical formation. Mol Chem Neuropathol. 1992;17(1):51–63. doi: 10.1007/BF03159981. [DOI] [PubMed] [Google Scholar]
- 36.Swamy M, Sirajudeen KNS, Chandran G. Nitric oxide [NO] citrulline-NO cycle enzymes, glutamin synthetase and oxidative status in kainic acid-mediated excitotoxicity in rat brain. Drug Chem Toxicol. 2009;32(4):326–331. doi: 10.1080/01480540903130641. [DOI] [PubMed] [Google Scholar]
- 37.Swamy M, Wan Roslina WY, Sirajudeen KNS, Zulkarnain M, Chandran G. Decreased glutamine synthetase, increased citrulline - nitric oxide cycle activities and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem. 2011;67(1):105–113. doi: 10.1007/s13105-010-0054-2. [DOI] [PubMed] [Google Scholar]
- 38.Swamy M, Wan Norlina WA, Suhaili Dian, Sirajudeen KNS, Zulkarnain M, Chandran G. Restoration of glutamine synthetase activity, nitric oxide levels and amelioration of oxidative stress by propolis in kainic acid mediated excitotoxicity. Afr J Trad CAM. 2014;11(2):458–463. doi: 10.4314/ajtcam.v11i2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ullah I, Park HY, Kim MO. Anthocyanins protect against kainic Acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci Ther. 2014;20(4):327–338. doi: 10.1111/cns.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- 41.White HS. Animal models of epileptogenesis. Neurology. 2002;59(9 suppl5):S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 42.Yang CW, Chen WL, Wu PL, Tseng HY, Lee SJ. Anti-inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol Pharmacol. 2006;69(3):749–758. doi: 10.1124/mol.105.017764. [DOI] [PubMed] [Google Scholar]
- 43.Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Ohkawa S, Ohnishi K, Terao S, Kawai C. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991;266(6):3369–3371. [PubMed] [Google Scholar]
- 44.Zheng X-Y, Zhang H-L, Luo Q, Zhu J. Kainic acid-induced neurodegenerative model. Potentials and limitations journal of biomedicine & biotechnology. 2011:457079. doi: 10.1155/2011/457079. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X M, Zhu J. Kainic acid-induced neurotoxicity: Targeting glial responses and glia-derived cytokines. Curr Neuropharmacol. 2011;9(2):388–398. doi: 10.2174/157015911795596540. [DOI] [PMC free article] [PubMed] [Google Scholar]