Abstract
Background
Propolis has been proposed to be protective on neurodegenerative disorders. To understand the neuroprotective effects of honeybee propolis, glutamine synthetase (GS) activity, nitric oxide (NO), thiobarbituric acid reactive substances (TBARS) and total antioxidant status (TAS) were studied in different brain regions-cerebral cortex (CC), cerebellum (CB) and brain stem (BS) of rats supplemented with propolis and subjected to kainic acid (KA) mediated excitotoxicity.
Materials and Methods
Male Sprague-Dawley rats were divided into four groups; Control group and KA group received vehicle and saline. Propolis group and propolis + KA group were orally administered with propolis (150mg/kg body weight), five times every 12 hours. KA group and propolis + KA group were injected subcutaneously with kainic acid (15mg/kg body weight) and were sacrificed after 2 hrs and CC, CB and BS were separated homogenized and used for estimation of GS activity, NO, TBARS, and TAS concentrations by colorimetric methods. Results were analyzed by one-way ANOVA, reported as mean + SD from 6 animals, and p<0.05 considered statistically significant.
Results
NO was increased (p< 0.001) and GS activity was decreased (p< 0.001) in KA treated group compared to control group as well as propolis + KA treated group. TBARS was decreased and TAS was increased (p< 0.001) in propolis + KA treated group compared KA treated group.
Conclusion
This study clearly demonstrated the restoration of GS activity, NO levels and decreased oxidative stress by propolis in kainic acid mediated excitotoxicity. Hence the propolis can be a possible potential candidate (protective agent) against excitotoxicity and neurodegenerative disorders.
Keywords: Nitric oxide, Glutamine Synthetase, Oxidative Stress, Excitotoxicity, Propolis, Rat Brain
Introduction
Glutamate and related excitatory amino acids are considered as major neurotransmitters in the central nervous system (CNS) and in that they are released by an estimated 40% of all synapses (Coyle and Puttafarcken, 1993). In addition to their ability to transmit vital excitatory CNS signals, they have shown to cause neuronal dysfunction by over stimulation of neurons. The ensuing excitotoxicity may be a causative factor in multitude of neurodegenerative diseases (Dawson et al., 1995; Dong et al., 2009). Astrocytes play a crucial role in regulating and maintaining the extracellular chemical milieu of the central nervous system under physiological conditions (Eid et al., 2013). In, the conversion of glutamate to glutamine by glutamine synthetase, that takes place within the astrocytes, represents a key mechanism in the regulation of excitatory neurotransmission under normal conditions as well as in injured brain (Szatkowski and Attwell, 1994). Thus GS is involved in modulation of the turnover of glutamate through the glutamate-glutamine cycle (Van der berg and Garfinkel, 1971). The known stoichiometry of glutamate transport across the astrocyte plasma membrane also suggests that rapid metabolism of intracellular glutamate via glutamine synthetase (GS) is a prerequisite for efficient glutamate clearance from the extracellular space (Eid et al., 2013).
Kainic acid (KA) is a potent CNS excitotoxin producing an acute and sub-acute epilepticform activity, ultimately resulting in wide spread irreversible neuropathological changes (Sperk, 1994). KA induced status epilepticus was associated with both apoptotic and necrotic cell death and induction of heat sensitive proteins in hippocampus and cortical regions of rodent brain (Akbar et al., 2001; Kato et al., 1999; White, 2002). The exact mechanisms contributing to increased concentration of nitric oxide (NO) in epilepsy are not well established. Earlier studies reported that nitric oxide synthase (NOS) knockout mice were more severely affected by epileptic activity than controls and the response to NO during epilepsy depends on its concentration (Itoh and Watanabe, 2009). It was also indicated that NO may be regarded as an anticonvulsant and proconvulsant substance in relation to convulsions induced by pentylenetetrazole (PTZ) (Itoh and Watanabe, 2009). Reactive Oxygen Species (ROS)/Reactive Nitrogen Species (RNS) have been implicated in the pathogenesis of various neurological disorders including epilepsy (Frantseva et al., 2000). Intracellular ROS are capable of inducing damage and, in severe cases, cell death through mitochondrial alterations leading to the release of cytochrome c (Berman and Hastings, 1999; Halestrap et al., 2000), through activation of the JNK pathway (Tournier et al., 2000) or by activation of nuclear factor-KB (NF-KB) transcription factors (Luo et al., 1999). The ability to control ROS is thus critical in neurodegenerative diseases, because neuronal damage occurs when the “oxidant-anti-oxidant” balances are disturbed in favor of excess oxidative stress (Maalouf et al., 2007). Stimulation of glutamate-KA receptors induces neuronal NO release, which in turn modulates glutamate transmission (Alabadi et al., 1999; Nakaki etal., 2000). NO induces changes in neuronal and signaling-related functions by several ways (Prast and Philippu, 2001).
Honey bee propolis has been widely used as a folk medicine and proposed to be protective on neurodegenerative disorders (Ha et al., 2010; Kwon et al., 2004). It has been shown to have broad biological activities, which are principally attributed to the presence of flavonoids (Isla et al., 2001) and caffeic acid phenyl ester (CAPE) (Natarajan et al., 1996). The prevailing opinion is that the broad biological activities of flavonoids and CAPE are related, in part, to their anti-inflammatory and anti oxidant actions (Isla et al., 2001; Natarajan et al., 1996). It was earlier reported that GS becomes nitrated and inhibited during PTZ induced seizure model at repeated PTZ seizure induction, but there was no decrease in GS protein level (Bidmon et al., 2008). Our earlier studies demonstrated that increased production of NO, increased activity of NOS, decreased activity of GS and increased oxidative stress in KA mediated excitotoxicity (Swamy et al., 2009, 2011a). Therefore the present study was conducted to assess the neuroprotective effects of the bee product propolis, by estimating the glutamine synthetase activity, nitric oxide (NO), thiobarbituric acid reactive substances (TBARS) concentration and total antioxidant status (TAS) in cerebral cortex (CC), cerebellum (CB) and brain stem (BS) of rats supplemented with propolis and subjected to KA mediated excitotoxicity.
Material and Methods
Propolis collection and ethanol extraction
Honey bee propolis was obtained from local bee products shop and it was subjected to 80% ethanol extract as per the procedure described by Isla et al. (2001).
Animals
Male Sprague Dawley rats weighing 200 – 250 grams were used for the study. The animals had free access to food and water. They were fed with commercial feed and had access to water ad libitum. They were housed under standard condition of constant temperature; humidity and a 12h light/dark cycle were maintained. Animal handling and experimental design was approved by the Animal ethics committee of Universiti Sains Malaysia, Health campus, Kubang Kerian, Malaysia [USM / Animal Ethics Approval / 20011 / (68) (296)].
Experimental Study
The rats were divided in to one control group and three study groups; KA group, propolis group and propolis + KA group. Control group and KA group received vehicle and saline. Propolis group and propolis + KA were orally administered with ethanol-extracted propolis (150mg/kg body weight), five times every 12 hours as described by Kwon et al. (2004). KA group and propolis + KA group rats were given subcutaneous injection of kainic acid (15mg/kg body weight) (Milatovic et al., 2002) and were sacrificed after 2hrs of KA injection. Control group and propolis group rats were given normal saline and sacrificed after 2hrs of saline injection.
After the rats sacrificed by decapitation the brain regions −CC, CB, and BS were separated according to the procedure described by Sadasivudu and Lajtha (1970). Each of the brain regions was weighed and used for the preparation of homogenates in 0.05M phosphate buffer pH 7.3.
Enzyme assay
GS activity was assayed by the method Rowe et al. (1970) as described by Swamy et al (2011a).
Estimations of NO, TBARS and TAS
NO was estimated as NOx (Nitrate/Nitrite) by Griess reaction after conversion of nitrate to nitrite by nitrate reductase, as described by Swamy et al (2011a) using the commercially available Nitric Oxide Assay Kit from Cayman Chemical Company (Catalogue number 780001; Ann Arbor, Michigan, USA). Lipid per oxidation was determined by the method of Chattered et al. (2000) by estimating TBARS as described by Swamy et al (2011a). TAS was estimated according to the method of Koracevic et al (2000) as described by Swamy et al (2011a).
Statistical analysis
Results were reported as mean + standard deviation (SD) from 6 animals for each parameter calculated. Statistical analysis of results was done by one-way analysis of variance (ANOVA) followed by post hoc analysis using Bonferroni's test, using the SPSS software (version 20) to determine the statistical significance of difference in values between the control and study groups. p value of < 0.05 was taken as statistically significant at 95% confidence interval.
Results
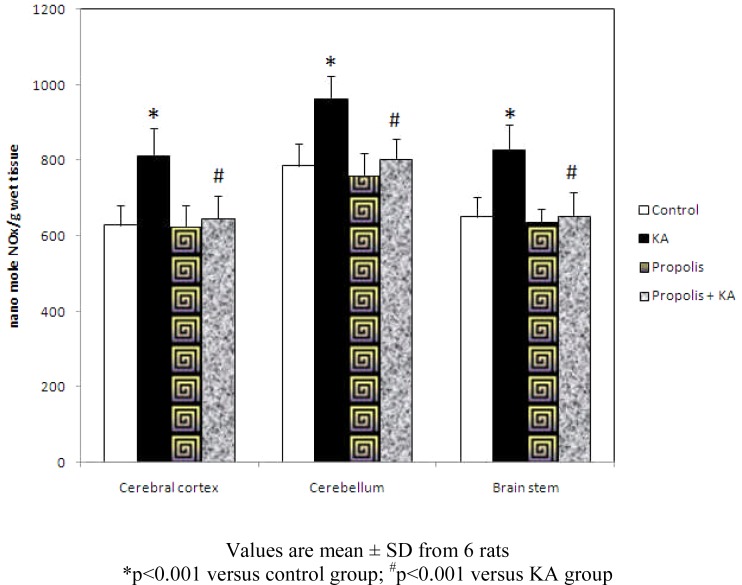
The concentration of NO was increased significantly (p<0.001) in all the three brain regions tested in KA group compared to control group, but the increase of NO concentration by KA was prevented by prior supplementation of propolis. There was no significant difference in NO level between control and propolis as well as propolis + KA group (Figure 1).
Figure 1.
Effect of propolis on concentration of NO in KA mediated excitotoxicity
Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
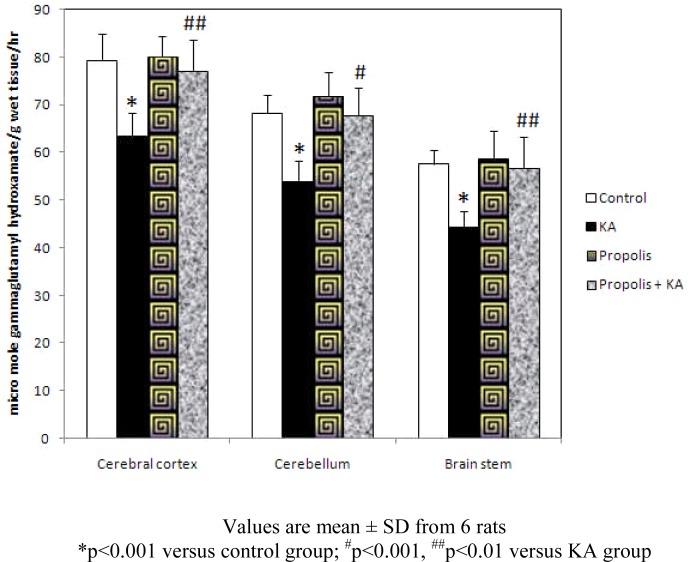
GS activity was decreased significantly (p<0.001) in all the three brain regions in KA group compared to control group and propolis + KA group indicating propolis treatment was preventing (p<0.001 in CB; p<0.01 in CC and BS) the GS activity decrease observed by KA treatment. There was no significant difference in GS activity between control and propolis as well as propolis + KA group propolis + KA group (Figure 2).
Figure 2.
Effect of propolis on activity of GS in KA mediated excitotoxicity
Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001, ##p<0.01 versus KA group
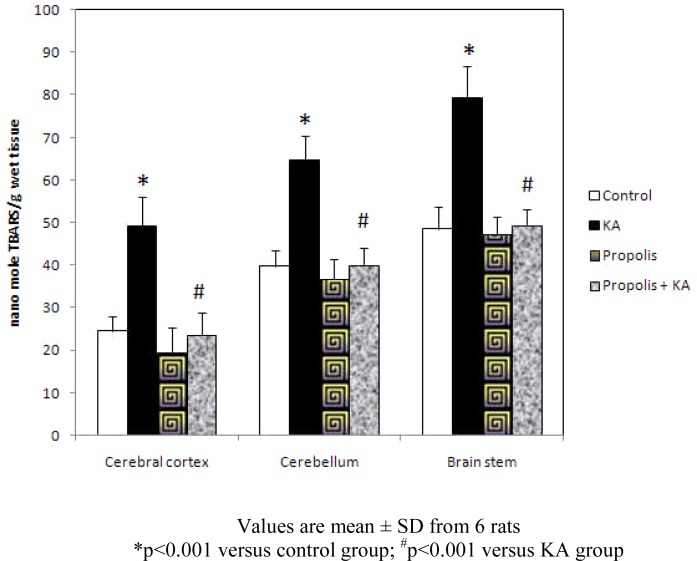
The concentration of TBARS was increased significantly (p<0.001) in all the three brain regions tested in KA group compared to control group, but the increase of TBARS concentration by KA was prevented (p<0.001) by prior supplementation with propolis (propolis + KA group). There was no significant difference in TBARS concentration between control and propolis as well as propolis + KA group (Figure 3).
Figure 3.
Effect of propolis on concentration of TBARS in KA mediated excitotoxicity
Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001 versus KA group
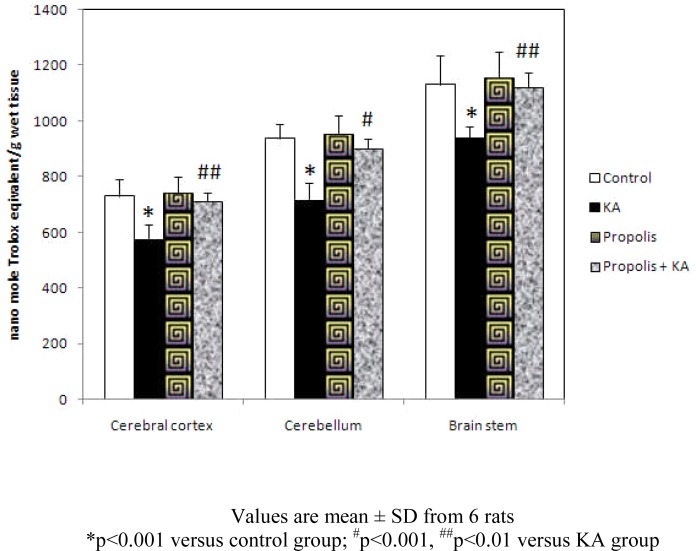
The concentration of TAS was decreased significantly (p<0.001) in KA group compared to control and propolis + KA group indicating the depletion of TAS concentration by KA was prevented (p<0.001 in CB; p<0.01 in CC and BS) by supplementation of propolis (propolis + KA group). There was no significant difference in TAS concentration between control and propolis as well as propolis + KA group propolis + KA group (Figure 4).
Figure 4.
Effect of propolis on concentration of TAS in KA mediated excitotoxicity
Values are mean ± SD from 6 rats
*p<0.001 versus control group; #p<0.001, ##p<0.01 versus KA group
Discussion
Glutamate, an excitatory amino acid, is one of the major neurotransmitter in the CNS. Evidences are shown that glutamate is highly neurotoxic when accumulated in high amount in the extra cellular space (Rothman and Olney, 1986; Takahashi et al., 1997). In the brain, the conversion of glutamate to glutamine by GS, that takes place within the astrocytes, represents a key mechanism in the regulation of excitatory neurotransmission (Szatkowski and Attwell, 1994). The glutamine synthetase activity is present in all parts of brain and it is equally high in cerebral cortex, cerebellum and hippocampus (Girard et al., 1993; Rose and Felipo 2005). The modulation of GS activity in brain, therefore, is important and its impairment or saturation may have pathological consequences (Rodrigo and Felipo 2007). Neuronal excitation involving the excitatory glutamate receptors is recognized as an important underlying mechanism in neurodegenerative disorders (Wang et al. 2005). Several studies have indicated that the activity of GS in astrocytes is diminished in several brain disorders, including epilepsy (Eid et al., 2012). Earlier studies have shown the decreased activity and expression of GS in kainic acid induced epilepsy and it has been hypothesized that the loss of GS activity in epilepsy leads to increased extracellular glutamate concentrations and epileptic seizures (Swamy et al., 2011a, b).
In neurons, NO synthesis is stimulated by Ca2+-influx, which is induced by activation of glutamate receptors, preferentially NMDA receptor (Radenovic and Selakovic, 2005). NO is known to be involved in the pathophysiology of many epilepsy models resulting from increased action of excitatory neurotransmitter namely glutamate (Lapouble et al., 2002; Penix et al., 1994; Rundfeldt et al., 1995). The literature findings implicate neuronal NO generation in the pathogenesis of both direct and secondary excitotoxic neuronal injuries in vivo. Although NMDA receptors likely contribute critically to neuronal injury in various acute conditions, several observations support the hypothesis that AMPA/KA receptors may be of greater importance to the neurodegenerative process (Carriedo et al., 1998, 2000).
Excitotoxicity and disrupted energy metabolism were considered to be acting in a synergistic manner leading to nerve cell death in neurodegenerative disorders (Dong et al., 2009). These cooperative pathways trigger oxidative stress by free radical formation (Silva-Adaya et al., 2008) and ROS/RNS are believed to cause lipid per oxidation with high levels of MDA resulting in damage to biological membranes (Chan, 2001). Epileptic form activity was shown to cause excessive production of ROS/RNS, a factor believed to be involved in the mechanisms leading to neurodegeneration and cell death (Itoh and Watanabe, 2009). The increased production of NO and increased oxidative stress in kainic acid mediated excitotoxicity and epilepsy has been reported earlier (Swamy et al., 2009, 2011a).
Propolis has been used to maintain health. Pharmacological activities such as anticancer, anti inflammatory, antibiotic, ant oxidative, antifungal, anesthetic and cytostatic have been ascribed to ethanolic extracts of propolis (Isla et al., 2001). Propolis has been shown to have broad biological activities, which are principally attributed to the presence of flavonoids (major component; rutin, quercetin, galangin, etc.), phenolic compounds and CAPE (Isla et al., 2001). The beneficial actions of propolis contents namely flavonoids, phenolic compounds and CAPE are related, in part, to their anti-inflammatory and anti oxidant actions (Isla et al., 2001; Kwon et al., 2004).
It has been reported that anti-inflammatory substances lucidone (Senthil Kumar et al., 2010), Curcumin (Jung et al., 2006), and phenantroindolizdine alkaloids (Yang et al., 2006) reduce NO production observed in inflammation. Though the active ingredients involved and mechanism is not known, the supplementation of propolis in this study showed the reduced production of NO in KA mediated excitotoxicity and may be attributed to anti-inflammatory and anti oxidant action of propolis. The decreased activity of GS in excitotoxicity attributed possible modulation by high concentration of NO was shown to be abolished by supplementation of propolis in this study. The results of the study clearly indicate that the supplementation of propolis shown the amelioration of oxidative stress caused by kainic acid in all the brain regions. Hence the supplementation of propolis may be beneficial to counteract the possible ways of excitotoxicity observed in many neurological disorders.
Conclusion
Results of this study clearly demonstrated the restoration of GS activity and NO levels along with decreased oxidative stress by propolis in kainic acid mediated excitotoxicity. Hence the propolis can be a possible potential candidate (protective agent) against excitotoxicity and neurodegenerative disorders.
Acknowledgements
This study received support from Universiti Sains Malaysia -Research University grant (A/C No: 1001/PPSP/813052). The findings of the study were presented in the International Conference on Medical & Health Sciences (ICMHS) 22–24th May 2013 at Renaissance Hotel, Kota Bharu, Malaysia and International symposium on Biological Engineering and Natural Science 2013 (ISBENS-2013), 26–28th July 2013 at Landmark Hotel, Bangkok.
References
- 1.Akbar MT, Wells DJ, Latchman DS, de Belleroche J. Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glail and neuronal cells compared to heat shock protein 70 and caspase 3 following kainite administrations. Brain Res Mol Brain Res. 2001;93(2):148–163. doi: 10.1016/s0169-328x(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 2.Alabadi J, Thibault JL, Pinard E, Seylaz J, Lasbennes F. 7-Nitroindazole a selective inhibitor of nNOS increases hippocampal extracellular glutamate concentration in status epilepticus induced by kainic acid in rats. Brain Res. 1999;839(2):305–312. doi: 10.1016/s0006-8993(99)01749-7. [DOI] [PubMed] [Google Scholar]
- 3.Bidmon HJ, Gorg B, Palomero-Gallagher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylenterazole model of epilepsy. Epilepsia. 2008;49(10):1733–1748. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 4.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria, implications for Parkinson's disease. J Neurochem. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 5.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. Rapid Ca2+entry through Ca2+ permeable AMPa/kainite channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci. 1998;1(19):7727–7738. doi: 10.1523/JNEUROSCI.18-19-07727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca2+overload and ROS generation in spinal motor neurons in vitro. J Neuroscience. 2000;20(1):240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowiski K, Stewart KN, Motafilipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58(2):658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 9.Coyle JT, Puttfarcken P. Oxidative stress, glutamate and neurodenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 10.Dawson R, Beal MF, Bondy SOC, DiMonte DA, Isom GE. Excitotoxins, aging, and environmental neurotoxins: Implications for understanding human neurodegenerative diseases. Toxicol Appl Pharm. 1995;134(1):1–17. doi: 10.1006/taap.1995.1163. [DOI] [PubMed] [Google Scholar]
- 11.Doble A. The role of excitotoxicity in neurodegenerative diseases implications for therapy. Pharmacol Ther. 1999;81(3):163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 12.Dong XX, Wang Y, Qin Z H. Molecular mechanisms of excitotoxicity and their relevance to pathogenisis of neurodegenerative diseases. Acta Pharmocol Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eid T, Behar K, Bumanglag AV, Lee TS. Role of glutamine synthetase inhibition in epilepsy. Neurochem Res. 2012;37(11):2339–2350. doi: 10.1007/s11064-012-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eid T, Tu N, Lee TS, Lai JOC. Regulation of glutamine synthetase in epilepsy. Neurochem Int. 2013;63(7):670–681. doi: 10.1016/j.neuint.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frantseva MV, Perez Velzquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, Carlen PL, Burnham MW. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience. 2000;97(3):431–435. doi: 10.1016/s0306-4522(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 16.Girard G, Giguere JF, Butterworth RF. Region selective reductions in activities of glutamine synthetase in rat brain following portacaval anastomosis. Metab Brain Dis. 1993;8(4):207–215. doi: 10.1007/BF01001062. [DOI] [PubMed] [Google Scholar]
- 17.Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010;485(3):143–147. doi: 10.1016/j.neulet.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP, Doran E, Gillespie JP, O'Toolee A. Mitochondria and cell death. Biochem Soc Trans. 2000;28(2):170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 19.Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001;76(2):165–170. doi: 10.1016/s0378-8741(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Watanabe M. Paradoxical facilitation of pentylenetetrazole-induced convulsion susceptibility in mice lacking neuronal nitric oxide synthase. Neuroscience. 2009;159(2):735–743. doi: 10.1016/j.neuroscience.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Jung KK, Lee HS, Cho JY, Shin WOC, Rhee MH, Kim TG, Kang JH, Kim SH, Hong S, Kang SY. Inhibitory effect of curcumin on nitric oxide production from lipopolisaccharide-activated primary microglia. Life Sci. 2006;79(21):2022–2031. doi: 10.1016/j.lfs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Katoh-Semba R, Takeuchi IK, Ito H, Kamei K. Responses of heat shock proteins hsp27, alphaB-crystalline, and hsp70 in rat brain after kainic acid-induced seizure activity. J Neurochem. 1999;73(1):229–236. doi: 10.1046/j.1471-4159.1999.0730229.x. [DOI] [PubMed] [Google Scholar]
- 23.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54(5):356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon YS, Park DH, Shin EJ, Kwon MS, Ko KH, Kim WK, Jhoo JH, Joo WK, Wie MB, Jung BD, Kim HOC. Antioxidant propolis attenuates Kainate-induced neurotoxicity Vai adenosine A1 receptor modulation in the rat. Neurosci Lett. 2004;355(3):231–235. doi: 10.1016/j.neulet.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 25.Lapouble E, Montecot C, Sevestre A, Pichon J. Phosphinothricin induces epileptic activity via nitric oxide production through NMDA receptor activation in adult mice. Brain Res. 2002;957(1):46–52. doi: 10.1016/s0006-8993(02)03597-7. [DOI] [PubMed] [Google Scholar]
- 26.Luo Y, Hattori A, Munoz J, Qin ZH, Roth GS. Intrastriatal dopamine injection induces apoptosis through oxidation-involved activation of transcription factors AP-1 and NK-kappaB in rats. Mol Pharmacol. 1999;56(2):254–264. doi: 10.1124/mol.56.2.254. [DOI] [PubMed] [Google Scholar]
- 27.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by ncreasing NADH oxidation. Neuroscience. 2007;145(1):256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milatovic D, Gupta ROC, Dettbarn WD. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002;957(2):330–337. doi: 10.1016/s0006-8993(02)03669-7. [DOI] [PubMed] [Google Scholar]
- 29.Nakaki T, Mishima A, Suzuki E, Shintani F, Fujii T. Glufosinate ammonium stimulates nitric oxide production through N-methyl-D-aspartate receptors in rat cerebellum. Neurosci Lett. 2000;290(3):209–212. doi: 10.1016/s0304-3940(00)01363-x. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan K, Singh S, Burke Jr TR, Grunberg D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kB. Proc Natl Acad Sci USA. 1996;93(17):9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penix LP, Davis W, Subramaniam S. Inhibition of NO synthase increases the severity of kainic acid-induced seizures in rodents. Epilepsy Res. 1994;18(3):177–184. doi: 10.1016/0920-1211(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 32.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64(1):51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 33.Radenovic L, Selakovic V. Differential effects of NMDA and AMPA/Kainate receptor antagonists on nitric oxide production in rat brain following intrahippocampal injection. Brain Res Bull. 2005;67(1–2):133–141. doi: 10.1016/j.brainresbull.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo R, Felipo V. Control of brain glutamine synthesis by NMDA receptors. Front Biosci. 2007;12:883–890. doi: 10.2741/2110. [DOI] [PubMed] [Google Scholar]
- 35.Rose C, Felipo V. Limited capacity for ammonia removal by brain in chronic liver failure: potential role of nitric oxide. Metab Brain Dis. 2005;20(4):275–283. doi: 10.1007/s11011-005-7906-4. [DOI] [PubMed] [Google Scholar]
- 36.Rothman SM, Olney JW. Glutamate and the pathophysiologyof hypoxic-ischemic brain damage. Ann Neurol. 1986;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 37.Rowe WB, Ronzio RA, Wellner VP, Meister A. Glutamine synthetase (Sheep brain) In: Tabor H, Tabor CW, editors. Methods in Enzymol. XVII Part A. New York: Academic Press; 1970. pp. 900–910. [Google Scholar]
- 38.Rundfeldt C, Koch R, Richter A, Mevissen M, Gerecke U, Loscher W. Dose-dependent anticonvulsant and proconvulsant effects of nitric oxide synthase inhibitors on seizure threshold in a cortical stimulation model in rats. Eur J Pharmacol. 1995;274(1–3):73–81. doi: 10.1016/0014-2999(94)00711-f. [DOI] [PubMed] [Google Scholar]
- 39.Sadasivudu B, Lajtha A. Metabolism of amino acids in incubated slices of mouse brain. J Neurochem. 1970;17(8):1299–1311. doi: 10.1111/j.1471-4159.1970.tb03379.x. [DOI] [PubMed] [Google Scholar]
- 40.Senthil Kumar KJ, Hsieh HW, Wang SY. Anti-inflammatory effect of lucidone in mice vai inhibition of NF-kappB?MPK kinase pathway. Int Immunopharmacol. 2010;10(4):385–392. doi: 10.1016/j.intimp.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Silva-Adaya D, Perez-De La Cruz V, Herrera-Mundo MN, Mendoza-Maccedo K, Villeda-Hernandez J, Bininda Z, Ali SF, Santamria A. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: antioxidant and neuroprotective effects of L-carnitine. J Neurochem. 2008;105(3):677–689. doi: 10.1111/j.1471-4159.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- 42.Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42(1):1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 43.Swamy M, Sirajudeen KNS, Chandran G. Nitric oxide [NO] citrulline-NO cycle enzymes, glutamins synthetase and oxidative status in kainic acid-mediated excitotoxicity in rat brain. Drug Chem Toxicol. 2009;32(4):326–331. doi: 10.1080/01480540903130641. [DOI] [PubMed] [Google Scholar]
- 44.Swamy M, Wan Roslina WY, Sirajudeen KNS, Zulkarnain M, Chandran G. Decreased glutamine synthetase, increased citrulline - nitric oxide cycle activities and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem. 2011a;67(1):105–113. doi: 10.1007/s13105-010-0054-2. [DOI] [PubMed] [Google Scholar]
- 45.Swamy M, Wan Roslina WY, Intan NMZ, Sirajudeen KNS, Zulkarnain M, Chandran G. Co-expression of citrulline - nitric oxide cycle enzymes and decreased glutamine synthetase expression in different regions of brain in epilepsy rat model. Afr J Pharm Pharmacol. 2011b;5(12):1522–1529. [Google Scholar]
- 46.Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17(9):359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M, Billups B, Rossi D, Sarantis M, Hamann M, Attwell D. The role of glutamate transporters in glutamate homeostasis in the brain. J Exp Biol. 1997;200(2):401–409. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- 48.Tournier C, Hes P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavella RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288(5467):870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 49.Van der berg CJ, Garfinkel D. A stimulation study of brain compartments, metabolism of glutamate and related substances in mouse brain. Biochem J. 1971;123(2):211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- 51.White HS. Animal models of epileptogenesis. Neurology. 2002;59(9 suppl 5):S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 52.Yang CW, Chen WL, Wu PL, Tseng HY, Lee SJ. Anti-inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol Pharmacol. 2006;69(3):749–758. doi: 10.1124/mol.105.017764. [DOI] [PubMed] [Google Scholar]