Abstract
Food webs are known to have myriad trophic links between resource and consumer species. While herbivores have well-understood trophic tendencies, the difficulties associated with characterizing the trophic positions of higher-order consumers have remained a major problem in food web ecology. To better understand trophic linkages in food webs, analysis of the stable nitrogen isotopic composition of amino acids has been introduced as a potential means of providing accurate trophic position estimates. In the present study, we employ this method to estimate the trophic positions of 200 free-roaming organisms, representing 39 species in coastal marine (a stony shore) and 38 species in terrestrial (a fruit farm) environments. Based on the trophic positions from the isotopic composition of amino acids, we are able to resolve the trophic structure of these complex food webs. Our approach reveals a high degree of trophic omnivory (i.e., noninteger trophic positions) among carnivorous species such as marine fish and terrestrial hornets.This information not only clarifies the trophic tendencies of species within their respective communities, but also suggests that trophic omnivory may be common in these webs.
Keywords: Carnivores, compound-specific isotope analysis, ecosystem, herbivores, omnivores, predators, primary producers, trophic position
Introduction
Recent studies have emphasized the importance of functional diversity in the provision of ecosystem services (Duffy et al. 2007; Griffin et al. 2008). Assessing the trophic niche of a species, however, has remained difficult, partly because there is little consensus as to appropriate metrics (Chase and Leibold 2003), and partly because there are so few empirical approaches that permit accurate and precise measurements of the feeding histories of animals (Chikaraishi et al. 2011; Steffan et al. 2013). This is particularly true for omnivores and higher-order consumers, where such groups are often left as large, undivided units rather than parsed into smaller trophic subsets (e.g., Polis and Strong 1996; Sih et al. 1998).
Evidence for the importance of omnivory in food webs has long been reported (e.g., Darnell 1961; Polis 1991; Coll and Guershon 2002; Bruno and O'Connor 2005). Indeed, multichannel omnivory has been postulated as a dominant feature of carnivore communities (Polis 1991; Polis and Strong 1996), with much subsequent support of this pattern (Rosenheim 1998; Coll and Guershon 2002; Williams and Martinez 2004; Finke and Denno 2005). Recent work suggests that species feeding above the level of strict herbivory are often a “tangled web” of trophic omnivores (Thompson et al. 2007), feeding opportunistically yet often expressing distinct trophic tendencies (Minagawa and Wada 1984; Power et al. 1985; Vander Zanden and Rasmussen 2001; Post 2002; Williams and Martinez 2004). These tendencies often exhibit characteristic variability (Jaksić and Delibes 1987; Bearhop et al. 2004), and such variation represents the “trophic spectrum” of a species (Polis and Strong 1996). Understanding trophic spectra may be critical to assessing the functional diversity of ecosystems, not only because the spectra provide information as to the variability, or range of trophic roles played by consumer species, but also because they indicate the central tendency of these species. Thus, measuring trophic spectra empirically should help tease apart the tangle of higher-order consumption by effectively characterizing the trophic niches of omnivores and carnivores.
Knowledge of the trophic position (TP) of organisms in food webs allows ecologists to track biomass flow, apportionment among trophic groups, and the trophic compositions of communities (e.g., Pimm 1991; Post 2002; Williams and Martinez 2004). Analysis of the stable nitrogen isotopic composition (δ15N) of amino acids represents a relatively new method that has been shown to provide accurate and precise estimates of the trophic position of organisms in aquatic and terrestrial systems (e.g., McClelland and Montoya 2002; McCarthy et al. 2007; Popp et al. 2007; Chikaraishi et al. 2009; Steffan et al. 2013). This approach is based on contrasting isotopic fractionation during metabolic processes between “trophic” and “source” amino acids (TrAAs and SrcAAs, respectively). For example, glutamic acid, a representative TrAA, shows significant 15N-enrichment (8.0‰ on average) during the transfer of biomass from one trophic level to another because its metabolism starts with transamination/deamination, which always cleaves carbon–nitrogen bonds (Fig. 1). Conversely, phenylalanine, a representative SrcAA, shows little 15N-enrichment (+0.4‰ on average) because its metabolism begins with the conversion of phenylalanine into tyrosine, which neither forms nor cleaves carbon–nitrogen bonds (Fig. 1). Thus, given the minimal enrichment of SrcAAs with each trophic transfer, the isotopic composition of SrcAAs in consumers represents the weighted average of all the resource species at the base of the food web. As an organism feeds higher in its food web, the δ15N value of TrAAs elevates predictably, while SrcAAs remains relatively static. A comparison of the isotopic composition between these two types of amino acids in any organism corresponds closely to the feeding position held by that organism within its food web (Steffan et al. 2013). In previous studies involving natural and laboratory-reared organisms, we established a general equation for the empirical measurement of an organism's trophic position:
Figure 1.
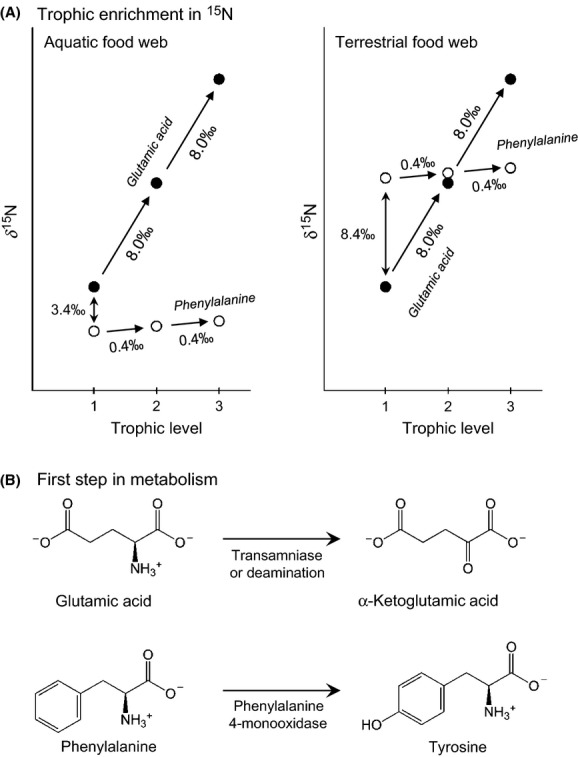
(A) Schematic illustration of the relationship between δ15N values of amino acids (glutamic acid and phenylalanine) and trophic level in food webs (after Chikaraishi et al. 2007, 2009), and (B) initial steps of the dominant metabolism for glutamic acid and phenylalanine in animals.
| (1) |
where the β represents the isotopic difference between glutamic acid (δ15NGlu) and phenylalanine (δ15NPhe) in primary producers (−3.4 ± 0.9‰ for aquatic cyanobacteria and algae, +8.4 ± 1.6‰ for terrestrial C3 plants, −0.4 ± 1.7‰ for terrestrial C4 plants), and the TDF represents trophic discrimination factor (7.6 ± 1.2‰ = Δ15NGlu − Δ15NPhe) at each shift of trophic level (Chikaraishi et al. 2010). Also, several previous studies used or suggested an alternative equation using a combination of all available isotopic composition (δ15N) of TrAAs and SrcAAs:
| (2) |
where the βTr/Src represents the isotopic difference between the weighted mean isotopic composition of TrAAs (δ15NTr) and SrcAAs (δ15NSrc) in primary producers, and the TDFTr/Src represents the TDF between TrAAs and SrcAAs (i.e., = Δ15NTr − Δ15NSrc) (e.g., Sherwood et al. 2011; Décima et al. 2013; Vander Zanden et al. 2013).
Using this method, the TP value is calculated as a linear function of the difference in the δ15N values of amino acids from the organism of interest (Chikaraishi et al. 2009; Steffan et al. 2013). As a result, the TP calculation accounts for the natural background variation in the nitrogen isotopic composition. In fact, previous studies reported that the standard deviation (1σ) of the accuracy of TPGlu/Phe value (= [actual TP] − [TPGlu/Phe]) was only 0.12 unit among aquatic species and 0.17 unit among terrestrial organisms, while the variability in the isotopic composition at the base of the food webs ranging up to ∼15‰ (Chikaraishi et al. 2009, 2011). The potential uncertainty in the TPGlu/Phe value calculated by taking into account the propagation of uncertainty on each factor in Eq. (1) is also only 0.23–0.24, 0.26–0.30, and 0.36–0.43 units for primary producers, primary consumers, and secondary consumers, respectively, in the terrestrial food web (Chikaraishi et al. 2011). This is a key advantage of this method and stands in contrast to traditional trophic position estimation techniques that rely on the nitrogen isotopic composition of bulk tissue samples (e.g., DeNiro and Epstein 1981; Minagawa and Wada 1984). The traditional bulk-analysis method is highly sensitive to background isotopic variation between the basal resources of a food web (e.g., Cabana and Rasmussen 1996; Vander Zanden et al. 1997; Vander Zanden and Rasmussen 1999; Post 2002). Another advantage of the amino acid approach is that it permits analyses of exceedingly small specimens (2 nmol for each amino acid, Chikaraishi et al. 2009), which allows researchers to assess the trophic functions of innumerable micro- and meso-fauna. Finally, the amino acid method is applicable to not only modern samples but also formalin-fixed and fossil (e.g., bone collagen) samples (Naito et al. 2010, 2013; Styring et al. 2010, 2012; Ogawa et al. 2013). Because of these advantages, the estimation of trophic position based on the isotopic composition of amino acids has been used with various organisms in recent ecological studies (e.g., McClelland et al. 2003; Hannides et al. 2009; Lorrain et al. 2009; Bloonfield et al. 2011; Dale et al. 2011; Sherwood et al. 2011; Maeda et al. 2012; Miller et al. 2012; Germain et al. 2013; Ruiz-Cooley et al. 2013; Vander Zanden et al. 2013).
However, the validity of this estimate is dependent on the consistency of both β and TDF values. Recent studies reported potentially little or substantial variation in the β value for cyanobacteria and algae (McCarthy et al. 2013), seagrass (Vander Zanden et al. 2013), and terrestrial C3 plants (Steffan et al. 2013). It was confirmed that the TDF value does not scale among trophic levels 1–4 in multiple controlled-feeding experiments and for trophic levels 1–5 in a natural food chain using terrestrial arthropod species (Steffan et al. 2013); however, the universality of the TDF has been questioned for several species, including penguins (Lorrain et al. 2009), elasmobranches (Dale et al. 2011), jumbo squids (Ruiz-Cooley et al. 2013), and harbor seals (Germain et al. 2013). In these species, small TDF values (3–5‰) were consistent with traditional biological observations such as stomach content analysis.
However, these biological observations did not involve empirical measurement of prey trophic position, and even if the prey trophic positions had been assayed, they would only have represented a snap-shot of the animal's feeding history. Thus, without lifelong measurements of prey trophic position, there is little basis to assert that TFDs of free-roaming marine species may be significantly different from the TDFs reported in controlled-feeding studies. Altogether, these results indicate that the β and TDF parameters are quite useful but would benefit from further refinement, particularly via controlled-feeding experiments involving various species, conditions, and positions within trophic hierarchies.
In the present study, we apply this method to investigations of selected flora and fauna in coastal marine (a stony shore) and terrestrial (a fruit farm) ecosystems in Japan. We aggregate data reported in previous studies (Chikaraishi et al. 2009, 2010, 2011) and report the TPGlu/Phe values of a total of 200 samples represented by 100 samples from 39 species in the coastal and 100 samples from 38 species in the terrestrial food webs (Table 1). Based on the observed TPGlu/Phe values, we illuminate elements of the food web structure in these ecosystems and further evaluate this new method of food web analysis.
Table 1.
Coastal marine and terrestrial organisms included in the present study
| Number of samples | Number of samples | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Ref 11 | Ref 21 | This study | Sample | Ref 21 | Ref 31 | This study |
| Marine costal (stony shore) | Terrestrial (fruit farm) | ||||||
| Macroalgae (Brown algae) | Plant | ||||||
| Undaria pinnatifida | 1 | 1 | Brassica oleracea | 3 | |||
| Sargassum filicinum | 2 | Daucus carota | 1 | ||||
| Ecklonia cava | 1 | Castanea crenata | 2 | 1 | |||
| Eisenia bicyclis | 1 | Citrus unshiu | 1 | ||||
| Macroalgae (Red algae) | Cucurbita moschata | 1 | |||||
| Binghamia californica | 1 | Diospyros kaki Thunberg | 1 | ||||
| Gelidium japonicum | 2 | 3 | Prunus avium | 1 | |||
| Gastropod | Raphanus sativus | 1 | |||||
| Batillus cornutus | 1 | 1 | Solanum lycopersicum | 1 | |||
| Haliotis discus | 1 | 1 | Solanum melongena | 1 | |||
| Omphalius pfeifferi | 1 | 4 | Solanum tuberosum | 1 | |||
| Echinoid | Aphid | ||||||
| Anthocidaris crassispina | 1 | Aphidoidea sp. | 1 | ||||
| Hemicentrotus pulcherrimus | 1 | Butterfly | |||||
| Oyster | Hestina assimilis | 1 | |||||
| Crassostrea sp. | 1 | Papilio machaon | 1 | ||||
| Crustacea | Papilio protenor | 1 | |||||
| Pachygrapsus crassipes | 1 | 1 | Pieris rapae (caterpillar) | 2 | 2 | ||
| Pagurus filholi | 1 | Pieris rapae | 2 | ||||
| Panulirus japonicus | 5 | Bee | |||||
| Plagusia dentipes | 1 | Apis mellifera | 3 | ||||
| Percnon planissimum | 1 | Bombus diversus diversus | 1 | 1 | |||
| Pugettia quadridens | 1 | Xylocopa appendiculata circumvolans | 1 | 1 | |||
| Thalamita pelsarti Montgomery | 1 | Katydid | |||||
| Fish | Gampsocleis mikado | 1 | |||||
| Acanthopagrus schlegeli | 1 | Holochlora japonica | 1 | ||||
| Apogon semilineatus | 11 | Paper wasp | |||||
| Canthigaster rivulata | 1 | Polistes japonicus japonicus | 6 | ||||
| Ditrema temmincki temmincki | 1 | Polistes jokahamae jokahamae | 3 | ||||
| Girella punctata | 1 | 14 | Polistes mandarinus | 1 | |||
| Gymnothorax kidako | 3 | Polistes rothneyi iwatai | 14 | ||||
| Goniistius zonatus | 1 | Parapolybia indica | 9 | ||||
| Halichoeres poecilopterus | 3 | Ant | |||||
| Lutjanus stellatus | 1 | Formica japonica | 1 | ||||
| Microcanthus strigatus | 3 | Ladybug | |||||
| Oplegnathus fasciatus | 2 | Coccinella septempunctata | 2 | ||||
| Oplegnathus punctatus | 1 | Harmonia axyridis | 3 | 4 | |||
| Parapristipoma trilineatum | 5 | Illeis koebelei | 5 | ||||
| Pseudoblennius percoides | 1 | Menochilus sexmaculatus | 2 | ||||
| Pseudolabrus siebold | 5 | Mantis | |||||
| Pteragogus flagellifer | 1 | Tenodera aridifolia | 1 | ||||
| Sebastes inermis | 2 | Hornet | |||||
| Sebastiscus marmoratus | 5 | Vespa analis fabriciusi | 7 | ||||
| Takifugu niphobles | 1 | Vespa ducalis pulchra | 3 | ||||
| Octopus | Vespa mandarinia japonica | 1 | 2 | ||||
| Octopus vulgaris | 1 | Vespa simillima xanthoptera | 1 | ||||
| Vespula flaviceps lewisii | 1 | ||||||
Materials and Methods
All of the marine and terrestrial samples were collected in 2001–2013 from a stony shore and a farm in Yugawara (35°08′N, 139°07′E), Japan, respectively. The stony shoreline surveyed represented ∼0.2 hectares and ranged in depth from 0 to 5 m, where brown and red macroalgae are dominant primary producers but seagrass is absent. The farm was also approximately 0.2 hectares with cultivation of fruits and vegetables, all of which were C3 plants. Green leaves and/or nuts were collected for higher plants, and whole samples of 1–15 individuals within a single stage were collected for the other species. The collected samples were cleaned with distilled water to remove surface contaminants and stored at −20°C. For most terrestrial species and marine macroalgae, whole-organism samples were prepared for isotopic analyses. For the remaining marine specimens, small samples of muscle tissue were taken. Shell samples were taken from several gastropod and lobster specimens, and scales were dissected from most of the fish species (Appendices A1 and A2). There was no substantial effect on the trophic position estimates among these different tissue types within a single animal specimen (e.g., Chikaraishi et al. 2010, 2011; Ogawa et al. 2013). The bulk-carbon and bulk-nitrogen isotopic compositions of representative samples (40 coastal marine and 69 terrestrial samples, Appendices A1 and A2) were determined using a Flash EA (EA1112) instrument coupled to a DeltaplusXP IRMS instrument with a ConFlo III interface (Thermo Fisher Scientific, Bremen, Germany). Carbon and nitrogen isotopic compositions are reported in the standard delta (δ) notation relative to the Vienna Peedee Belemnite (VPDB) and to atmospheric nitrogen (AIR), respectively.
The nitrogen isotopic composition of amino acids was determined by gas chromatography/combustion/isotope ratio mass spectrometry (GC/C/IRMS) after HCl hydrolysis and N-pivaloyl/isopropyl (Pv/iPr) derivatization, according to the procedure in Chikaraishi et al. (2009) (which are described in greater detail at http://www.jamstec.go.jp/biogeos/j/elhrp/biogeochem/download_e.html). In brief, samples were hydrolyzed using 12 Mol/L HCl at 110°C. The hydrolysate was washed with n-hexane/dichloromethane (3/2, v/v) to remove hydrophobic constituents. Then, derivatizations were performed sequentially with thionyl chloride/2-propanol (1/4) and pivaloyl chloride/dichloromethane (1/4). The Pv/iPr derivatives of amino acids were extracted with n-hexane/dichloromethane (3/2, v/v). The nitrogen isotopic composition of amino acids was determined by GC/C/IRMS using a 6890N GC (Agilent Technologies, Palo Alto, CA) instrument coupled to a DeltaplusXP IRMS instrument via a GC-C/TC III interface (Thermo Fisher Scientific, Bremen, Germany). To assess the reproducibility of the isotope measurement and obtain the amino acid isotopic composition, reference mixtures of nine amino acids (alanine, glycine, leucine, norleucine, aspartic acid, methionine, glutamic acid, phenylalanine, and hydroxyproline) with known δ15N values (ranging from −25.9‰ to +45.6‰, Indiana University, SI science co.) were analyzed after every four to six samples runs, and three pulses of reference N2 gas were discharged into the IRMS instrument at the beginning and end of each chromatography run for both reference mixtures and samples. The isotopic composition of amino acids in samples was expressed relative to atmospheric nitrogen (AIR) on scales normalized to known δ15N values of the reference amino acids. The accuracy and precision for the reference mixtures were always 0.0‰ (mean of Δ) and 0.4–0.7‰ (mean of 1σ) for sample sizes of ≥1.0 nmol N, respectively.
The δ15N values were determined for the following 10 amino acids: alanine, glycine, valine, leucine, isoleucine, proline, serine, methionine, glutamic acid, and phenylalanine (Appendices A1 and A2). These amino acids were chosen because their peaks were always well separated with baseline resolution in the chromatogram (Chikaraishi et al. 2009). Also, it should be noted that glutamine was quantitatively converted to glutamic acid during acid hydrolysis; as a result, the α-amino group of glutamine contributed to the δ15N value calculated for glutamic acid.
The TPGlu/Phe value (and its potential uncertainty calculated by taking into account the propagation of uncertainty on each factor in the Eq. (1)) was calculated from the observed δ15N values (as 1σ = 0.5‰) of glutamic acid and phenylalanine in the organisms of interest, using eq. (1) with the β value of −3.4 ± 0.9‰ for coastal marine and +8.4 ± 1.6‰ for terrestrial samples, and with the TDF value of 7.6 ± 1.2‰ for both ecosystems, according to Chikaraishi et al. (2009, 2010, 2011). The TPTr/Scr values were not calculated, because we did not measure the δ15N values of lysine and tyrosine for all investigated samples and of serine for approximately a half of samples.
Results and Discussion
δ13C and δ15N values of bulk samples
Carbon and nitrogen isotopic compositions of bulk samples ranged from −17.7‰ to −8.7‰ and from +4.8‰ to +14.2‰, respectively, within the coastal marine system (Appendix A1). In the terrestrial system, respective carbon and nitrogen isotopic compositions ranged from −32.5‰ to −21.8‰ and from −2.8‰ to +9.1‰ (Appendix A2). These two ecosystems are readily distinguished in the δ13C-δ15N cross-plot of the organisms, mainly because of disparity in the δ13C value of the food web resource between coastal marine and terrestrial systems (Fig. 2).
Figure 2.
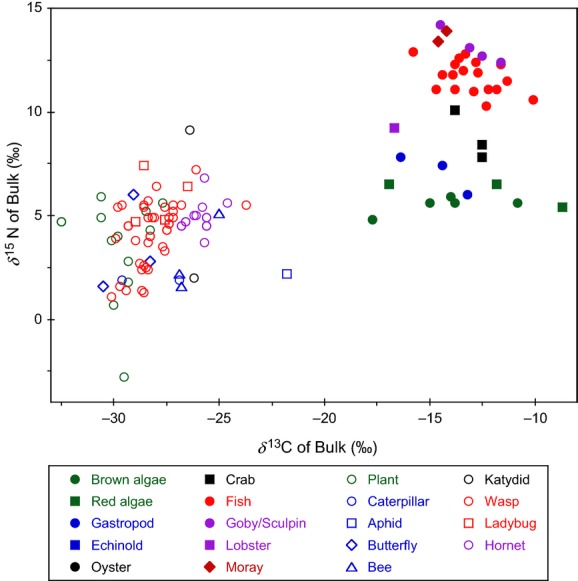
δ13C and δ15N values of bulk samples.
In the present study, the nitrogen isotopic composition ranges from +4.8‰ to +7.8‰ for marine algae and from −2.8‰ to +5.9‰ for the terrestrial plants. This heterogeneity in the isotopic composition of basal resources, particularly in the terrestrial system, was relatively large up to 2.6 times as large as the discrimination factor (i.e., 3.4‰; Minagawa and Wada 1984), which is used to estimate the trophic position based on bulk isotopic composition.
Precision of TPGlu/Phe for multiple sample analysis
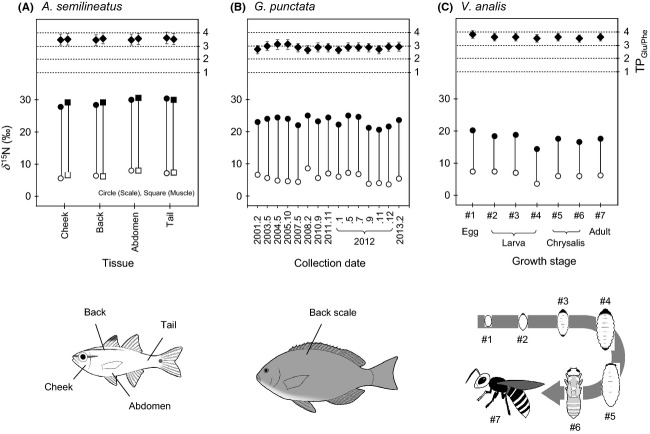
Based on the analysis of 5–15 individuals within a single stage for 11 representative species (i.e., eight coastal marine and three terrestrial organisms, Table 2), we first evaluated natural variation in the TPGlu/Phe value for the investigated organisms. As summarized in Table 2, the standard deviation for the comparison of the TPGlu/Phe values and an average of potential uncertainty in the TPGlu/Phe value calculated by taking into account the propagation of uncertainty on each factor in eq. (1) were always less than 0.13 and 0.46 for coastal marine and less than 0.11 and 0.24 for terrestrial organisms. These were almost identical to the precision levels previously reported for the TPGlu/Phe value (Chikaraishi et al. 2009, 2011). As shown in Fig. 3A, there was a quite small difference in the TPGlu/Phe value (1σ = 0.06 for the comparison of the TPGlu/Phe values) among scale and muscle collected from cheek, back, abdomen, and tail within a single sample of the fish Apogon semilineatus, although the δ15N values of phenylalanine are different, ranging up to 2.4‰ among body parts and 1.1‰ between tissue types. A small difference (1σ = 0.13) was also found between 17 individuals of the fish Girella punctata collected from this coastal area over a decade during 2001–2013, although its phenylalanine has a variation in the δ15N value ranging up to 5.0‰ during this term (Fig. 3B).
Table 2.
The estimated TPGlu/Phe values of 5–17 individuals within a single stage for 11 representative species
| TPGlu/Phe | |||||
|---|---|---|---|---|---|
| Sample | Number of samples | Average | 1σ1 | 1σ2 | |
| Red algae | Gelidium japonicum | 5 | 1.07 | 0.11 | 0.15 |
| Gastropod | Omphalius pfeifferi | 5 | 2.01 | 0.09 | 0.22 |
| Crustacea | Polistes japonicus | 5 | 3.86 | 0.09 | 0.46 |
| Fish | Apogon semilineatus | 11 | 3.53 | 0.05 | 0.42 |
| Fish | Girella punctata | 15 | 2.88 | 0.13 | 0.33 |
| Fish | Parapristipoma trilineatum | 5 | 2.91 | 0.06 | 0.33 |
| Fish | Pseudolabrus siebold | 5 | 3.32 | 0.07 | 0.39 |
| Fish | Sebastiscus marmoratus | 5 | 4.06 | 0.13 | 0.50 |
| Paper wasp | Polistes rothneyi | 6 | 3.02 | 0.09 | 0.24 |
| Ladybug | Harmonia axyridis | 5 | 3.06 | 0.07 | 0.24 |
| Ladybug | Illeis koebelei | 5 | 3.05 | 0.11 | 0.24 |
Standard deviation (1σ) for the comparison of the TPGlu/Phe values from multiple samples.
An average of potential uncertainly in TPGlu/Phe value calculated by taking into account the propagation of 1σ for δ15NGlu, δ15NPhe, β, and TDF in eq. (1).
Figure 3.
δ15N values of glutamic acid and phenylalanine and the TPGlu/Phe values for (A) difference parts (cheek, back, abdomen, and tail) and tissues (scale and muscle) within a single fish Apogon semilineatus, (B) different individuals of a fish Girella punctata collected during 2001–2013, and (C) different growth stages of a hornet Vespa analis. Bar represents potential uncertainly in TPGlu/Phe calculated by taking into account the propagation of 1σ for δ15NGlu, δ15NPhe, β, and TDF in eq. (1).
Secondly, we evaluated the effect of metamorphosis on the TPGlu/Phe value from the egg to adult stages of terrestrial insect species. We investigated this because the feeding pattern and appearance of many holometabolous insects show a marked change during metamorphosis. As summarized in Table 3, the standard deviation (1σ) for the comparison of the TPGlu/Phe values was always less than 0.14 units for seven terrestrial insect species including herbivore (butterfly) and carnivores (paper wasps, ladybug, and hornet). Interestingly, a small change in the TPGlu/Phe value (1σ = 0.11) between different stages is commonly found even in the hornet Vespa analis, an opportunistic predator (they can feed on many insects; Takamizawa 2005). The constancy in the TPGlu/Phe value of this hornet was evident despite the fact that there were marked differences (between 3.6 and 7.4‰) in the δ15N values of phenylalanine at different growth stages, which represent temporal changes in the diet of this hornet family (Fig. 3C). These results reveal how a consumer's trophic position can remain unchanged during a given period of time, even though its food type and/or source has changed dramatically.
Table 3.
Standard deviation (1s) of the estimated TPGlu/Phe values of seven representative terrestrial species with different growth stages
| N | TPGlu/Phe | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Egg | Larva | Chrysalis | Adult | Total | Average | 1σ1 | 1σ2 | |
| Butterfly | Pieris rapae | 0 | 4 | 0 | 2 | 6 | 2.09 | 0.14 | 0.24 |
| Paper wasp | Polistes japonicus | 1 | 2 | 2 | 1 | 6 | 3.02 | 0.14 | 0.24 |
| Paper wasp | Polistes jokahamae | 1 | 1 | 0 | 1 | 3 | 3.07 | 0.14 | 0.24 |
| Paper wasp | Polistes rothneyi | 1 | 3 | 5 | 4 | 13 | 3.03 | 0.14 | 0.24 |
| Paper wasp | Parapolybia indica | 0 | 3 | 4 | 2 | 9 | 2.97 | 0.11 | 0.24 |
| Ladybug | Harmonia axyridis | 0 | 1 | 1 | 5 | 6 | 3.07 | 0.06 | 0.24 |
| Hornet | Vespa analis | 1 | 3 | 2 | 1 | 7 | 3.05 | 0.11 | 0.29 |
Standard deviation (1σ) for the comparison of the TPGlu/Phe values from multiple samples.
An average of potential uncertainly in TPGlu/Phe value calculated by taking into account the propagation of 1σ for δ15NGlu, δ15NPhe, β, and TDF in eq. (1).
Mapping of food webs using trophic isoclines
Using equation (1), the δ15N values for phenylalanine and glutamic acid can be plotted against each other, creating a line for each trophic position with slope of 1.0, and between-line interval of 7.6‰ (Fig. 4). All points within each line are the algebraic solutions for the parameter of the isotopic composition of glutamic acid, while holding the trophic position constant and substituting into the equation a range of phenylalanine δ15N values. Each line therefore represents a trophic isocline (or a “trophocline”), and altogether, these lines demarcate the trophic levels of a food web in 2-dimensional phase space. In this space, the trophic position of organisms can be plotted according to their respective δ15N values of glutamic acid and phenylalanine. One of the advantages of this graphical presentation is that background heterogeneity in the isotopic composition is completely transparent (evident as the δ15N value of phenylalanine along the horizontal axis). Whatever the δ15N values of phenylalanine in an organism are, the δ15N value of glutamic acid will reflect its trophic position. When the TPGlu/Phe values of organisms are arrayed across trophoclines in phase space, it becomes apparent how populations simultaneously vary in terms of trophic position and background δ15N values (e.g., Chikaraishi et al. 2009). For example, the isotopic composition of phenylalanine is highly variable in the coastal marine and terrestrial ecosystems (the δ15N values ranging from 3.5 to 8.7‰ and from 1.6 to 17.0‰, respectively). Despite this high level of background heterogeneity, all of the algal and higher plant samples have the TPGlu/Phe values that were on or near the line of TPGlu/Phe = 1 (Fig. 4), within the precision levels (e.g., 0.15 unit for aquatic algae and 0.30–0.36 unit for terrestrial plants, as potential uncertainty in the TPGlu/Phe value) in coastal marine (χ2 = 49.994, df = 11, P = 1.000) and terrestrial environments (χ2 = 64.330, df = 14, P = 1.000). Furthermore, the species known to be herbivores, such as the gastropods, caterpillars, and bees, all were plotted on the TPGlu/Phe = 2 line within the precision levels (e.g., 0.19–0.22 unit for aquatic and 0.23–0.25 unit for terrestrial organisms, as potential uncertainty in the TPGlu/Phe value) in coastal marine (χ2 = 70.314, df = 10, P = 1.000) and terrestrial environments (χ2 = 54.757, df = 18, P = 1.000).
Figure 4.
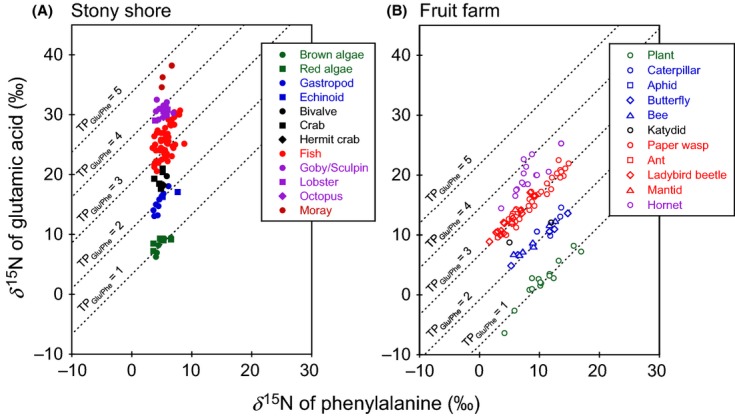
Cross-plots for δ15N values of glutamic acid and phenylalanine for (A) coastal marine and (B) terrestrial ecosystems. The potential propagation uncertainly is 0.15 for brown and red macroalgae, 0.19–0.22 for gastropod and echinoid, 0.25–0.29 for bivalve, crab, and hermit crab, 0.30–0.42 for fish, 0.43–0.53 for goby/sculpin, lobster, and octopus, 0.55–0.59 for moray, 0.30–0.36 for plant, 0.23–0.25 for caterpillar, aphid, butterfly, and bee, 0.23–0.24 for katydid, 0.23–0.26 for paper wasp, ant, ladybird beetle, and mantid, and 0.27–0.33 for hornet.
Importantly, the array of data points in this phase space could reveal linear food chains within the broader food web. Considering that the TDF value for phenylalanine is only 0.4 ± 0.5‰ (Chikaraishi et al. 2009), the δ15N values of phenylalanine in a consumer closely reflect those of all the resources (e.g., Chikaraishi et al. 2009). In other words, consumer and resource species arrayed in vertical columns within a narrow range of the δ15N values of phenylalanine could represent highly compartmentalized and linear food webs, whereas a species that registers a wide range of the δ15N value of phenylalanine could indicate a consumer that can exploit resources from multiple communities, ecosystems, or bioregions. Also, all consumer species falling within a range of δ15N values for phenylalanine may effectively “belong” to a single particular food web. In fact, in the present study, the δ15N values of phenylalanine of the algae in the coastal marine system ranged from 3.6 to 6.6‰, which corresponds very closely to the range found in coastal marine consumers (from 3.5 to 8.7‰) (Fig. 4). In the terrestrial system, the δ15N values of phenylalanine in plants ranged from 4.1 to 17.0‰, which was more variable but nevertheless corresponded closely to the range found in terrestrial consumers (1.6 to 14.9‰) (Fig. 4). These results suggest that the consumer species of each ecosystem had likely fed principally on the local resources and thus were derived from these particular food webs.
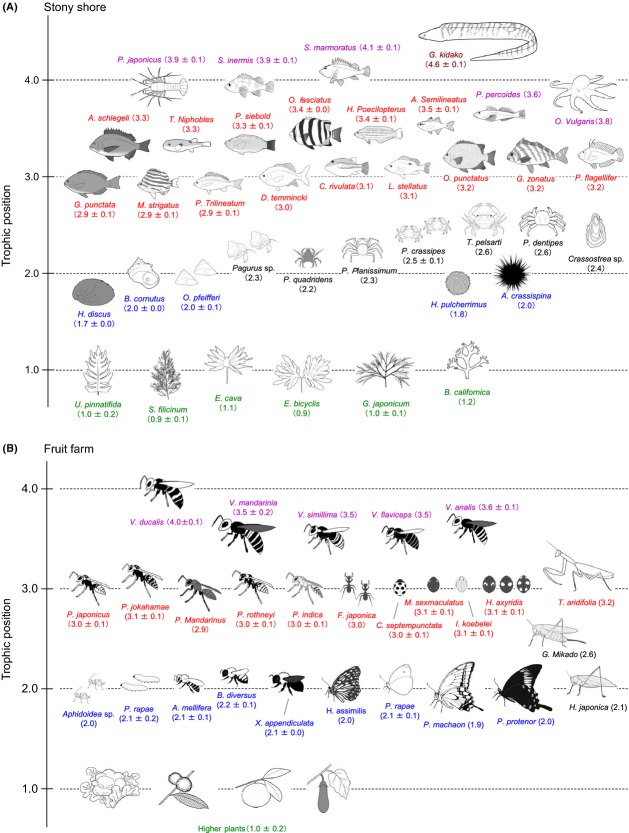
Most food chains start with primary producers (TP = 1) such as algae and plants, which are eaten by herbivores (strict plant-feeders: TP = 2) and omnivores (both plant- and animal-feeders: TP > 2). Herbivores and omnivores are eaten by carnivores (animal-feeders: TP > 3) and finally by tertiary predators (carnivores at the top of the food chain). Based on the observed TPGlu/Phe values, we can effectively map subsets of the communities within coastal marine (Fig. 5A) and terrestrial ecosystems (Fig. 5B). Marine primary producers were represented by macroalgae with TPGlu/Phe values ranging from 0.9 to 1.2. As expected, gastropods and echinoids registered as herbivores, given TPGlu/Phe values of 1.7 to 2.0. Various crabs and bivalves (i.e., oysters) appear to be omnivores, as their TPGlu/Phe values range from 2.2 to 2.6. On the other hand, fish and lobsters have a large variation in the TPGlu/Phe values, ranging from 2.9 to 4.6, revealing a high degree of trophic omnivory within this group. The moray eel (Gymnothorax kidako) appears to be a top predator with a TPGlu/Phe value of 4.6 in this environment.
Figure 5.
Illustration of food web structure in (A) the coastal marine and (B) terrestrial ecosystems. Mean trophic position and 1σ for the comparison of the observed TPGlu/Phe values in each species are shown in a parenthesis under each organism.
In the farm ecosystem (Fig. 5B), higher plants had TPGlu/Phe values ranging from 0.7 to 1.3. The data are consistent with the ecologically expected trophic positions for aphids (Aphidoidea sp., TPGlu/Phe = 2.0), caterpillars (Pieris rapae, TPGlu/Phe = 2.1), bees (e.g., Apis mellifera, TPGlu/Phe = 2.1), butterflies (e.g., P. rapae, TPGlu/Phe = 2.1), and herbivorous katydids (Holochlora japonica, TPGlu/Phe = 2.1), all of which are known herbivores. Gampsocleis mikado, a katydid species known to be an omnivorous scavenger (e.g., ElEla et al. 2010), registered a TPGlu/Phe value of 2.6. Paper wasps (e.g., Polistes japonicusm TPGlu/Phe = 3.0), ants (Formica japonica, TPGlu/Phe = 3.0), ladybird beetles (e.g., Coccinella septempunctata, TPGlu/Phe = 3.0), and mantids (Tenodera aridifolia, TPGlu/Phe = 3.2) are secondary consumers with TPGlu/Phe values ranging from 2.9 to 3.2. The TPGlu/Phe values of hornets (e.g., V. analis and Vespa ducalis) ranged from 3.5 to 4.0.
Trophic omnivory among carnivorous species can be measured as the degree to which consumers’ trophic positions depart from an integer-based trophic position (i.e., trophic level 3.0, 4.0). For example, the mean TPGlu/Phe value of carnivorous/omnivorous fish was 3.33 ± 0.47, which was significantly different from trophic level 3.0 (one-sample t-test: t = 5.59, df = 62, P < 0.001) or 4.0 (t = −11.34, df = 62, P < 0.001). The value of hornets was 3.64 ± 0.06), which was significantly different from either trophic level 3.0 (t = 11.45, df = 14, P < 0.001) or 4.0 (t = −6.44, df = 14, P < 0.001).
In the present study, the trophic position was calculated using eq. (1) with the β value of −3.4‰ for coastal marine and +8.4‰ for terrestrial samples and with the TDF value of 7.6‰ for both ecosystems, according to Chikaraishi et al. (2010). On the other hand, recent studies also reported potential variation in the β and TDF values for several species, which may leads under- or over-estimation of the trophic position of organisms by up to 2.0 unit (e.g., Germain et al. 2013; Vander Zanden et al. 2013). However, it seems to be that the β and TDF values reported in Chikaraishi et al. (2010) are applicable in the studied food webs. In fact, the estimated TPGlu/Phe values of primary producers (i.e., macroalgae and plants) and herbivores (e.g., gastropods and caterpillars) were always close to 1.0 and 2.0, respectively, within the precision levels (Fig. 5). The TPGlu/Phe values of wasps (2.9–3.0) and a hornet V. ducalis (4.0) are particularly consistent with the biologically expected trophic positions that the wasps feed primarily on caterpillars found on plant leaves and this hornet feeds solely on wasps (e.g., Takamizawa 2005).
Implications
In the traditional approach to the trophic position estimation using bulk δ15N values of organisms, substantial background heterogeneity in the isotopic composition often causes significant uncertainty in the mapping of food web structure (e.g., Cabana and Rasmussen 1996; Vander Zanden et al. 1997; Post 2002). The present study demonstrates that δ15N analysis of individual amino acids can attend to background heterogeneity while simultaneously allowing precise estimation of the trophic positions of free-roaming organisms. As predicted by theory and early empirical work (Polis 1991; Polis and Strong 1996), the trophic structure evident in the marine and terrestrial systems we studied are indicative of multichannel omnivory: A number of the animal species registered noninteger trophic levels. Our data therefore represent evidence of the ubiquity of trophic omnivory in marine and terrestrial ecosystems. Plotting the trophic spectra of these species across trophoclines reveals the degree of omnivory (Fig. 5). Accommodating background heterogeneity and trophic position simultaneously will allow researchers to assess compartmentalization within a food web while also assessing the trophic niche breadth of populations and communities.
Dual isotope analysis using nitrogen (δ15N) and carbon (δ13C) in bulk samples has widely been used for the food web structure analysis in a number of previous studies (e.g., Cabana and Rasmussen 1996; Yoshii et al. 1999; Aita et al. 2011). In these studies, ideally, the δ15N values provide trophic position estimates of organisms because of the significant enrichment in 15N with each trophic level (by ∼3‰ at each level; DeNiro and Epstein 1981; Minagawa and Wada 1984), whereas the δ13C values directly provide diet resources of organisms because of relatively small enrichment along the trophic level (by ∼1‰ at each level; DeNiro and Epstein 1978). Although the carbon isotope analysis of amino acids is still under development (e.g., Corr et al. 2007; Smith et al. 2009; Dunn et al. 2011), little or no trophic enrichment in 13C was commonly found in the essential amino acids in controlling feeding experiments (e.g., Hare et al. 1991; O'Brien et al. 2002; Howland et al. 2003; McMahon et al. 2010). Moreover, the δ13C values in the essential AAs potentially provide taxonomic (e.g., among bacteria, fungi, microalgae, seagrasses, and terrestrial plants; Larsen et al. 2009, 2013) and geographical discrimination among food sources (McMahon et al. 2012). Accordingly, it is expected that the combination of accurate trophic position estimates (using δ15N values of amino acids) with accurate food source estimates (using δ13C values of amino acids) will be potentially useful for better understanding the complex networks of multiple food chains.
Acknowledgments
We thank Mr. Mikiyo Chikaraishi (Yamani farm) for providing samples and Ms. Rutsu Hirono for drawing illustrations of organisms. This work was supported by Grant-in-Aid for Scientific Research of the JSPS (Y.C., N.O.O., M.T., and N.O.) and USDA-ARS appropriated funds to SAS (3655-21220-001-00D).
Appendix A1: Nitrogen isotopic composition of amino acids in coastal marine organisms
| δ15N1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Tissue | Collection Date | δ13CBulk | Bulk | Alanine | Glycine | Valine | Leucine | Isoleucine | Proline | Serine | Methionine | Glutamic acid | Phenylalanine | TPGlu/Phe2 | 1σ3 | Source4 |
| Macroalgae (Brown algae) | |||||||||||||||||
| Undaria pinnatifida (#1) | Whole | 2001.2 | −10.8 | 5.6 | 8.0 | −0.7 | 6.8 | 5.1 | 5.7 | 5.4 | −2.1 | 2.0 | 6.9 | 4.1 | 0.9 | 0.2 | Ref 1 |
| Undaria pinnatifida (#2) | Whole | 2006.5 | −13.8 | 5.6 | 7.7 | 3.2 | 8.3 | 8.1 | 7.2 | 6.7 | −0.3 | 2.7 | 9.2 | 4.7 | 1.1 | 0.2 | Ref 2 |
| Sargassum filicinum (#1) | Whole | 2001.2 | −15.0 | 5.6 | 7.3 | −1.5 | 8.4 | 2.8 | 5.8 | 7.2 | −1.6 | 2.0 | 6.3 | 4.1 | 0.8 | 0.2 | Ref 1 |
| Sargassum filicinum (#2) | Whole | 2006.4 | −14.0 | 5.9 | 7.9 | −0.8 | 9.3 | 4.6 | 7.2 | 9.0 | 1.6 | 2.5 | 8.2 | 4.4 | 1.0 | 0.2 | Ref 1 |
| Ecklonia cava | Whole | 2010.9 | −17.7 | 4.8 | 8.2 | 2.7 | 9.4 | 7.9 | 7.8 | 9.2 | 2.0 | 4.6 | 9.3 | 5.4 | 1.1 | 0.2 | This study |
| Eisenia bicyclis | Whole | 2010.9 | n.d. | n.d. | 9.3 | 3.1 | 10.0 | 9.1 | 8.2 | 8.8 | −1.6 | 3.8 | 9.4 | 6.5 | 0.9 | 0.2 | This study |
| Macroalgae (Red algae) | |||||||||||||||||
| Binghamia californica | Whole | 2001.2 | −11.8 | 6.5 | 6.9 | −0.8 | 8.3 | 4.9 | 6.4 | 7.3 | −1.4 | 0.9 | 8.6 | 3.6 | 1.2 | 0.2 | Ref 1 |
| Gelidium japonicum (#1) | Whole | 2001.2 | −16.9 | 6.5 | 8.2 | 2.9 | 7.7 | 5.7 | 7.0 | 7.3 | −3.3 | 3.2 | 9.3 | 4.7 | 1.2 | 0.2 | Ref 1 |
| Gelidium japonicum (#2) | Whole | 2005.5 | n.d. | n.d. | 8.3 | 0.6 | 6.5 | 5.7 | 6.2 | 6.4 | −2.5 | 3.5 | 9.1 | 5.2 | 1.1 | 0.2 | This study |
| Gelidium japonicum (#3) | Whole | 2006.5 | −12.8 | 7.3 | 10.4 | 0.4 | 10.6 | 8.5 | 9.4 | 10.5 | 0.6 | 3.8 | 9.2 | 6.6 | 0.9 | 0.2 | Ref 1 |
| Gelidium japonicum (#4) | Whole | 2010.9 | −8.7 | 5.4 | 8.9 | 1.5 | 6.9 | 7.0 | 5.9 | 5.0 | n.d. | n.d. | 7.3 | 3.7 | 1.0 | 0.2 | This study |
| Gelidium japonicum (#5) | Whole | 2012.11 | n.d. | n.d. | 7.4 | −0.5 | 5.1 | 4.4 | 8.0 | 8.8 | n.d. | n.d. | 9.1 | 5.1 | 1.1 | 0.2 | This study |
| Gastropod | |||||||||||||||||
| Batillus cornutus (#1) | Muscle | 2001.2 | −16.4 | 7.8 | 16.6 | 4.8 | 15.1 | 12.7 | 14.3 | 14.1 | 7.0 | 3.1 | 16.4 | 5.0 | 2.0 | 0.2 | Ref 1 |
| Batillus cornutus (#2) | Shell | 2001.2 | n.d. | n.d. | 16.2 | 3.9 | 14.9 | 12.9 | 14.7 | n.d. | 7.7 | n.d. | 15.9 | 4.6 | 2.0 | 0.2 | This study |
| Haliotis discus (#1) | Muscle | 2001.2 | −13.2 | 6.0 | 12.6 | 2.8 | 12.7 | 6.1 | 9.5 | 12.9 | −0.6 | 1.9 | 13.2 | 4.3 | 1.7 | 0.2 | Ref 1 |
| Haliotis discus (#2) | Shell | 2001.2 | n.d. | n.d. | 12.7 | 2.6 | 12.4 | 5.5 | 9.3 | 12.7 | 0.1 | n.d. | 13.1 | 3.8 | 1.8 | 0.2 | This study |
| Omphalius pfeifferi (#1) | Muscle | 2001.2 | −14.4 | 7.4 | 15.1 | 3.6 | 11.4 | 8.9 | 9.0 | 11.7 | 2.4 | 2.5 | 14.7 | 4.4 | 1.9 | 0.2 | Ref 1 |
| Omphalius pfeifferi (#2) | Shell | 2001.2 | n.d. | n.d. | 15.4 | 3.0 | 11.7 | 8.5 | 9.8 | 11.1 | 3.3 | n.d. | 15.0 | 4.0 | 2.0 | 0.2 | This study |
| Omphalius pfeifferi (#3) | Muscle | 2006.4 | n.d. | n.d. | 15.2 | 5.1 | 13.5 | 12.3 | 16.7 | 13.0 | 4.1 | 4.3 | 18.0 | 6.1 | 2.1 | 0.2 | This study |
| Omphalius pfeifferi (#4) | Muscle | 2010.11 | n.d. | n.d. | 13.6 | 2.5 | 13.8 | 11.2 | 12.6 | 13.3 | 4.1 | 1.7 | 14.0 | 3.6 | 1.9 | 0.2 | This study |
| Omphalius pfeifferi (#5) | Muscle | 2010.11 | n.d. | n.d. | 17.0 | 5.0 | 16.1 | 14.2 | 15.1 | 15.9 | 7.4 | 2.9 | 16.8 | 5.2 | 2.1 | 0.2 | This study |
| Echinoid | |||||||||||||||||
| Anthocidaris crassispina | Shell | 2010.11 | n.d. | 5.8 | 15.5 | 6.8 | 16.0 | 13.0 | 12.8 | n.d. | n.d. | 5.5 | 16.2 | 5.2 | 2.0 | 0.2 | This study |
| Hemicentrotus pulcherrimus | Shell | 2010.11 | n.d. | 7.3 | 17.6 | 8.3 | 15.5 | 14.3 | 13.8 | n.d. | n.d. | n.d. | 17.1 | 7.6 | 1.8 | 0.2 | This study |
| Bivalve | |||||||||||||||||
| Crassostrea sp. | Muscle | 2010.11 | n.d. | 8.3 | 18.4 | 10.1 | 21.2 | 16.7 | 18.3 | 17.4 | 11.4 | n.d. | 19.7 | 5.8 | 2.4 | 0.3 | This study |
| Crustacean | |||||||||||||||||
| Pachygrapsus crassipes (#1) | Muscle | 2001.2 | −12.5 | 7.8 | 15.9 | 3.7 | 15.4 | 10.7 | 9.4 | 15.4 | 3.3 | 1.9 | 19.3 | 3.8 | 2.6 | 0.3 | Ref 1 |
| Pachygrapsus crassipes (#2) | Muscle | 2012.7 | n.d. | n.d. | 17.0 | 3.1 | 14.7 | 14.3 | 13.7 | n.d. | n.d. | n.d. | 18.5 | 4.6 | 2.4 | 0.3 | This study |
| Pagurus filholi | Muscle | 2012.7 | n.d. | n.d. | 16.0 | 3.2 | 13.2 | 13.0 | 10.5 | n.d. | 7.5 | 3.0 | 18.2 | 5.3 | 2.3 | 0.2 | This study |
| Panulirus japonicus (#1) | Sell | 2011.1 | −16.7 | 9.2 | 25.4 | 12.1 | 23.3 | 21.4 | 21.0 | 13.8 | 5.2 | n.d. | 29.1 | 3.9 | 3.9 | 0.5 | This study |
| Panulirus japonicus (#2) | Sell | 2011.1 | n.d. | n.d. | 25.5 | 6.2 | 23.0 | 19.8 | 18.8 | 15.4 | 7.1 | n.d. | 30.9 | 6.0 | 3.8 | 0.5 | This study |
| Panulirus japonicus (#3) | Sell | 2011.1 | n.d. | n.d. | 25.8 | 11.2 | 22.1 | 21.8 | 22.3 | 13.3 | 5.8 | n.d. | 29.9 | 5.8 | 3.7 | 0.4 | This study |
| Panulirus japonicus (#4) | Sell | 2011.1 | n.d. | n.d. | 23.0 | 7.6 | 22.6 | 23.9 | 21.4 | 11.3 | 4.7 | n.d. | 31.0 | 5.1 | 4.0 | 0.5 | This study |
| Panulirus japonicus (#4) | Sell | 2012.5 | n.d. | n.d. | 27.2 | 8.8 | 26.1 | 24.3 | 22.2 | 11.5 | 3.3 | n.d. | 30.6 | 5.2 | 3.9 | 0.5 | This study |
| Plagusia dentipes | Muscle | 2001.2 | −13.8 | 10.1 | 14.9 | 6.0 | 16.3 | 13.0 | 9.9 | 16.9 | 6.4 | 2.6 | 20.4 | 5.1 | 2.6 | 0.3 | Ref 1 |
| Percnon planissimum | Muscle | 2001.2 | −12.5 | 8.4 | 14.1 | 7.5 | 12.8 | 12.9 | 12.3 | 16.4 | 6.6 | 1.8 | 17.9 | 4.9 | 2.3 | 0.2 | Ref 1 |
| Pugettia quadridens | Muscle | 2013.4 | n.d. | n.d. | 17.4 | 4.7 | 15.4 | 7.9 | 8.0 | 16.4 | 2.2 | n.d. | 21.0 | 5.1 | 2.6 | 0.3 | This study |
| Thalamita pelsarti Montgomery | Muscle | 2013.4 | n.d. | n.d. | 16.4 | 2.1 | 13.3 | 11.3 | 13.9 | 13.8 | 0.7 | n.d. | 17.7 | 4.8 | 2.2 | 0.2 | This study |
| Fish | |||||||||||||||||
| Acanthopagrus schlegeli | Scale | 2007.5 | −12.2 | 11.1 | 20.0 | 7.4 | 19.9 | 19.4 | 21.5 | 21.9 | 11.2 | 2.4 | 25.6 | 4.9 | 3.3 | 0.4 | Ref 1 |
| Apogon semilineatus (#1) | Scale | 2012.5 | −12.7 | 11.9 | 25.5 | 7.5 | 22.6 | 23.6 | 21.7 | 21.2 | n.d. | n.d. | 26.4 | 3.8 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#2) | Scale | 2012.5 | n.d. | n.d. | 25.9 | 6.7 | 24.2 | 22.9 | 19.5 | 23.1 | n.d. | n.d. | 27.0 | 4.2 | 3.6 | 0.4 | This study |
| Apogon semilineatus (#3) | Scale | 2012.5 | n.d. | n.d. | 27.2 | 8.8 | 25.5 | 24.2 | 23.2 | 22.5 | n.d. | n.d. | 27.4 | 4.2 | 3.6 | 0.4 | This study |
| Apogon semilineatus (#4–1) | Scale | 2012.5 | n.d. | n.d. | 27.0 | 7.2 | 24.6 | 23.4 | 20.5 | 24.5 | 10.6 | 4.4 | 27.7 | 5.5 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#4–2) | Scale | 2012.5 | n.d. | n.d. | 26.4 | 6.9 | 26.2 | 23.4 | 23.5 | 22.5 | n.d. | n.d. | 28.4 | 6.4 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#4–3) | Scale | 2012.5 | n.d. | n.d. | 24.7 | 6.1 | 24.3 | 22.4 | 23.6 | 24.7 | n.d. | n.d. | 30.1 | 7.9 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#4–4) | Scale | 2012.5 | n.d. | n.d. | 25.3 | 6.6 | 23.6 | 24.4 | 20.6 | 26.7 | 9.1 | 6.4 | 30.4 | 7.1 | 3.6 | 0.4 | This study |
| Apogon semilineatus (#4–5) | Muscle | 2012.5 | n.d. | n.d. | 25.5 | 8.5 | 24.8 | 22.8 | 24.3 | 24.0 | 4.1 | 4.5 | 29.1 | 6.6 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#4–6) | Muscle | 2012.5 | n.d. | n.d. | 25.5 | 8.0 | 24.7 | 19.9 | 21.5 | 24.9 | 3.5 | 4.9 | 29.1 | 6.1 | 3.6 | 0.4 | This study |
| Apogon semilineatus (#4–7) | Muscle | 2012.5 | n.d. | n.d. | 24.3 | 7.6 | 22.4 | 18.3 | 23.9 | 23.1 | 6.5 | 4.6 | 30.7 | 8.0 | 3.5 | 0.4 | This study |
| Apogon semilineatus (#4–8) | Muscle | 2012.5 | n.d. | n.d. | 24.6 | 6.1 | 22.2 | 19.1 | 25.7 | 25.9 | 5.7 | 3.3 | 30.0 | 7.3 | 3.5 | 0.4 | This study |
| Canthigaster rivulata | Muscle | 2012.11 | −13.8 | 12.3 | 21.2 | 2.1 | 19.8 | 21.0 | 20.9 | n.d. | n.d. | n.d. | 25.5 | 6.1 | 3.1 | 0.4 | This study |
| Ditrema temmincki temmincki | Scale | 2012.11 | −12.9 | 11.0 | 21.6 | 6.0 | 22.3 | 17.7 | 18.1 | 22.5 | n.d. | n.d. | 23.8 | 5.5 | 3.0 | 0.3 | This study |
| Girella punctata (#1) | Scale | 2001.2 | n.d. | n.d. | 19.3 | 6.5 | 22.4 | 16.4 | 19.7 | 19.3 | n.d. | n.d. | 23.1 | 6.6 | 2.7 | 0.3 | This study |
| Girella punctata (#2) | Scale | 2003.5 | n.d. | n.d. | 19.6 | 6.2 | 21.8 | 19.3 | 18.7 | 22.5 | n.d. | n.d. | 24.0 | 5.6 | 3.0 | 0.3 | This study |
| Girella punctata (#3) | Scale | 2004.5 | n.d. | n.d. | 19.4 | 6.3 | 22.4 | 16.5 | 20.0 | 23.2 | n.d. | n.d. | 24.4 | 4.9 | 3.1 | 0.4 | This study |
| Girella punctata (#4) | Scale | 2005.10 | n.d. | n.d. | 19.2 | 6.3 | 21.8 | 19.0 | 19.3 | 21.7 | n.d. | n.d. | 24.0 | 4.7 | 3.1 | 0.4 | This study |
| Girella punctata (#5) | Scale | 2007.5 | −13.8 | 11.1 | 20.7 | 6.3 | 20.2 | 18.1 | 19.8 | 19.4 | 11.5 | 1.6 | 22.0 | 4.4 | 2.9 | 0.3 | Ref 1 |
| Girella punctata (#6) | Scale | 2008.2 | n.d. | n.d. | 19.5 | 6.5 | 21.1 | 18.6 | 22.0 | 19.2 | n.d. | n.d. | 25.1 | 8.7 | 2.7 | 0.3 | This study |
| Girella punctata (#7) | Scale | 2010.9 | −13.9 | 11.8 | 23.5 | 8.2 | 26.2 | 24.6 | 24.5 | 25.4 | 3.9 | 4.4 | 23.2 | 5.6 | 2.9 | 0.3 | This study |
| Girella punctata (#8) | Scale | 2011.11 | −14.7 | 11.1 | 24.2 | 7.9 | 22.4 | 19.9 | 19.3 | 23.2 | 9.6 | 6.4 | 24.5 | 7.0 | 2.9 | 0.3 | This study |
| Girella punctata (#9) | Scale | 2012.1 | n.d. | n.d. | 20.2 | 7.0 | 21.0 | 19.7 | 18.6 | 18.5 | n.d. | n.d. | 22.2 | 6.0 | 2.7 | 0.3 | This study |
| Girella punctata (#10) | Scale | 2012.5 | n.d. | n.d. | 19.5 | 7.6 | 20.7 | 19.1 | 20.3 | 19.2 | n.d. | n.d. | 25.0 | 7.2 | 2.9 | 0.3 | This study |
| Girella punctata (#11) | Scale | 2012.7 | n.d. | n.d. | 17.9 | 10.5 | 20.0 | n.d. | 19.3 | n.d. | n.d. | n.d. | 24.7 | 6.8 | 2.9 | 0.3 | This study |
| Girella punctata (#12) | Scale | 2012.9 | n.d. | n.d. | 21.2 | 7.5 | 22.2 | 27.3 | 16.5 | 15.4 | 9.7 | 1.6 | 21.3 | 3.7 | 2.9 | 0.3 | This study |
| Girella punctata (#13) | Scale | 2012.11 | n.d. | n.d. | 20.0 | 9.6 | 18.7 | 15.5 | 12.1 | 18.4 | n.d. | 2.2 | 20.5 | 4.1 | 2.7 | 0.3 | This study |
| Girella punctata (#14) | Scale | 2012.12 | n.d. | n.d. | 20.4 | 8.7 | 17.0 | 16.9 | 19.8 | 20.0 | n.d. | 1.5 | 21.7 | 3.7 | 2.9 | 0.3 | This study |
| Girella punctata (#15) | Scale | 2013.2 | n.d. | n.d. | 16.6 | 4.9 | 19.2 | 18.9 | 20.0 | n.d. | n.d. | n.d. | 23.5 | 5.4 | 2.9 | 0.3 | This study |
| Gymnothorax kidako (#1) | Muscle | 2011.11 | −14.2 | 13.9 | 36.2 | 10.3 | 34.1 | 27.1 | 31.3 | 31.5 | 5.8 | 2.6 | 36.2 | 5.2 | 4.6 | 0.6 | This study |
| Gymnothorax kidako (#2) | Muscle | 2012.2 | −14.6 | 13.4 | 32.5 | 9.8 | 33.1 | 32.3 | 38.3 | 15.5 | n.d. | 4.1 | 38.2 | 6.6 | 4.7 | 0.6 | This study |
| Gymnothorax kidako (#3) | Muscle | 2012.11 | n.d. | n.d. | 28.5 | 7.6 | 28.2 | 25.4 | 37.3 | 29.1 | n.d. | 2.7 | 34.6 | 5.0 | 4.4 | 0.6 | This study |
| Goniistius zonatus | Scale | 2012.11 | −12.3 | 10.3 | 21.0 | 7.9 | 22.2 | 17.6 | 19.2 | 23.1 | n.d. | 5.4 | 26.2 | 6.1 | 3.2 | 0.4 | This study |
| Halichoeres poecilopterus (#1) | Scale | 2010.9 | −11.6 | 12.3 | 21.4 | 11.0 | 20.6 | 20.0 | 11.6 | 25.6 | n.d. | n.d. | 27.0 | 5.3 | 3.4 | 0.4 | This study |
| Halichoeres poecilopterus (#2) | Scale | 2011.12 | n.d. | n.d. | 23.7 | 7.5 | 24.7 | 22.9 | 22.9 | 27.6 | n.d. | 5.5 | 28.3 | 7.2 | 3.3 | 0.4 | This study |
| Halichoeres poecilopterus (#3) | Scale | 2011.12 | n.d. | n.d. | 23.7 | 9.5 | 24.5 | 22.6 | 18.0 | 25.7 | n.d. | 6.4 | 29.6 | 7.1 | 3.5 | 0.4 | This study |
| Lutjanus stellatus | Scale | 2012.11 | −10.1 | 10.6 | 21.9 | 9.0 | 21.3 | 16.3 | 16.5 | 21.5 | n.d. | 4.7 | 25.2 | 6.0 | 3.1 | 0.4 | This study |
| Microcanthus strigatus (#1) | Scale | 2011.11 | −13.4 | 12.0 | 20.6 | 7.0 | 22.9 | 22.2 | 22.0 | 20.8 | 11.8 | 4.6 | 24.1 | 5.3 | 3.0 | 0.3 | This study |
| Microcanthus strigatus (#2) | Scale | 2012.1 | n.d. | n.d. | 22.4 | 7.2 | 22.9 | 24.0 | 21.1 | 22.7 | n.d. | n.d. | 22.8 | 5.8 | 2.8 | 0.3 | This study |
| Microcanthus strigatus (#3) | Scale | 2012.7 | n.d. | n.d. | 21.5 | 19.5 | 21.5 | 20.1 | 19.7 | 18.7 | n.d. | 3.9 | 22.4 | 5.0 | 2.8 | 0.3 | This study |
| Oplegnathus fasciatus (#1) | Scale | 2012.8 | −13.3 | 12.8 | 23.9 | 3.6 | 21.5 | 20.0 | 19.1 | n.d. | 8.9 | 3.3 | 25.1 | 3.9 | 3.3 | 0.4 | This study |
| Oplegnathus fasciatus (#2) | Scale | 2012.8 | −13.6 | 12.6 | 27.0 | 9.6 | 23.6 | 25.2 | 22.3 | n.d. | 12.4 | 5.0 | 28.2 | 6.6 | 3.4 | 0.4 | This study |
| Oplegnathus punctatus | Scale | 2012.9 | −11.8 | 11.1 | 18.6 | 6.3 | 18.0 | 13.6 | 14.1 | 12.7 | 10.6 | 3.3 | 24.1 | 4.0 | 3.2 | 0.4 | This study |
| Parapristipoma trilineatum (#1) | Scale | 2012.9 | −12.8 | 12.4 | 21.7 | 8.1 | 20.0 | 16.8 | 12.7 | 14.4 | 10.5 | 3.2 | 22.3 | 4.5 | 2.9 | 0.3 | This study |
| Parapristipoma trilineatum (#2) | Scale | 2012.9 | n.d. | n.d. | 22.1 | 8.3 | 17.5 | 15.7 | 12.0 | 11.6 | 11.5 | 4.8 | 23.1 | 5.7 | 2.8 | 0.3 | This study |
| Parapristipoma trilineatum (#3) | Scale | 2012.9 | n.d. | n.d. | 21.3 | 8.9 | 19.5 | 13.6 | 10.2 | 20.6 | 9.5 | 3.9 | 22.3 | 4.1 | 2.9 | 0.3 | This study |
| Parapristipoma trilineatum (#4) | Scale | 2012.9 | n.d. | n.d. | 22.4 | 8.9 | 20.6 | 16.4 | 19.5 | 18.7 | 12.8 | 4.2 | 23.4 | 5.8 | 2.9 | 0.3 | This study |
| Parapristipoma trilineatum (#5) | Scale | 2012.9 | n.d. | n.d. | 22.8 | 8.4 | 20.3 | 13.9 | 13.3 | 17.8 | 11.8 | 2.9 | 24.0 | 5.4 | 3.0 | 0.3 | This study |
| Pseudoblennius percoides | Muscle | 2011.12 | −14.5 | 14.2 | 27.7 | 7.7 | 28.8 | 25.0 | 29.6 | 29.5 | n.d. | 5.6 | 30.2 | 6.9 | 3.6 | 0.4 | This study |
| Pseudolabrus siebold (#1) | Scale | 2011.11 | −11.3 | 11.5 | 21.0 | 8.2 | 21.4 | 17.6 | 18.7 | 22.4 | 11.2 | 2.4 | 24.4 | 3.5 | 3.3 | 0.4 | This study |
| Pseudolabrus siebold (#2) | Scale | 2011.12 | n.d. | n.d. | 23.6 | 10.2 | 24.3 | 22.1 | 22.3 | 25.2 | 15.2 | 4.9 | 26.8 | 5.9 | 3.3 | 0.4 | This study |
| Pseudolabrus siebold (#3) | Scale | 2011.12 | n.d. | n.d. | 23.1 | 8.9 | 25.7 | 22.6 | 18.6 | n.d. | n.d. | n.d. | 25.8 | 5.3 | 3.2 | 0.4 | This study |
| Pseudolabrus siebold (#4) | Scale | 2011.12 | n.d. | n.d. | 24.2 | 8.7 | 26.3 | 24.0 | 21.9 | 23.8 | n.d. | n.d. | 25.8 | 4.9 | 3.3 | 0.4 | This study |
| Pseudolabrus siebold (#5) | Scale | 2013.2 | n.d. | n.d. | 24.7 | 7.9 | 24.7 | 25.7 | 21.3 | n.d. | n.d. | n.d. | 26.9 | 4.9 | 3.4 | 0.4 | This study |
| Pteragogus flagellifer | Scale | 2011.12 | −14.4 | 11.8 | 26.4 | 12.9 | 21.6 | 17.8 | n.d. | 24.5 | n.d. | 5.6 | 26.0 | 5.7 | 3.2 | 0.4 | This study |
| Sebastes inermis (#1) | Scale | 2012.5 | −12.5 | 12.5 | 28.4 | 6.4 | 30.5 | 24.3 | 27.4 | 31.6 | 8.9 | 3.2 | 31.6 | 5.3 | 4.0 | 0.5 | This study |
| Sebastes inermis (#2) | Scale | 2013.2 | n.d. | n.d. | 23.2 | 3.0 | 25.8 | 19.3 | 23.6 | 30.2 | 2.8 | n.d. | 29.2 | 4.4 | 3.8 | 0.5 | This study |
| Sebastiscus marmoratus (#1) | Scale | 2012.1 | −13.1 | 13.1 | 29.4 | 8.9 | 27.9 | 28.4 | 29.6 | 30.1 | 13.7 | 1.8 | 30.6 | 4.0 | 4.0 | 0.5 | This study |
| Sebastiscus marmoratus (#2) | Scale | 2012.1 | −11.6 | 12.4 | 30.1 | 7.1 | 28.1 | 31.1 | 30.7 | 31.4 | 11.9 | 3.8 | 32.6 | 4.2 | 4.3 | 0.5 | This study |
| Sebastiscus marmoratus (#3) | Scale | 2012.1 | n.d. | n.d. | 31.7 | 7.8 | 28.0 | 32.6 | 26.9 | 30.0 | 13.5 | 4.9 | 32.1 | 5.8 | 4.0 | 0.5 | This study |
| Sebastiscus marmoratus (#4) | Scale | 2012.1 | n.d. | n.d. | 28.2 | 7.5 | 26.2 | 29.9 | 25.6 | 28.5 | n.d. | n.d. | 30.9 | 4.5 | 4.0 | 0.5 | This study |
| Sebastiscus marmoratus (#5) | Scale | 2013.2 | n.d. | n.d. | 28.1 | 7.8 | 29.3 | 22.7 | 24.9 | n.d. | n.d. | n.d. | 31.5 | 5.7 | 3.9 | 0.5 | This study |
| Takifugu niphobles | Muscle | 2010.9 | −15.8 | 12.9 | 24.9 | 11.5 | n.d. | 26.0 | 21.9 | n.d. | 17.4 | 3.5 | 26.2 | 5.7 | 3.3 | 0.4 | This study |
| Octopus | |||||||||||||||||
| Octopus vulgaris | Muscle | 2013.1 | n.d. | n.d. | 22.5 | 6.0 | 26.1 | 21.3 | 22.7 | n.d. | 1.0 | n.d. | 29.6 | 5.3 | 3.8 | 0.5 | This study |
n.d.: Not determined.
The δ15N value was determined by single analysis for each sample.
TPGlu/Phe = (δ15NGlu − δ15NPhe − 3.4)/7.6 + 1.
Propagation error on the TPGlu/Phe value based on 1σ on the δ15N measurement of amino acids in this study and 1σ on the β and TDF values reported in Chikaraishi et al. 2010.
Appendix 2: Nitrogen isotopic composition of amino acids in terrestrial organisms
| δ15N1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Stage | Tissue | Collection Date | δ13CBulk | Bulk | Alanine | Glycine | Valine | Leucine | Isoleucine | Proline | Serine | Methionine | Glutamic acid | Phenylalanine | TPGlu/Phe2 | 1σ3 | Source4 |
| Higher plant | ||||||||||||||||||
| Brassica oleracea (#1) | – | Leaf | 2008.11 | −32.5 | 4.7 | 0.2 | −6.7 | 5.1 | 3.8 | 3.9 | 9.5 | 1.0 | 0.8 | 5.7 | 13.1 | 1.1 | 0.3 | Ref 2 |
| Brassica oleracea (#2) | – | Leaf | 2011.11 | n.d. | n.d. | 1.7 | −7.0 | 3.7 | 0.7 | 3.4 | 4.6 | −2.1 | n.d. | 2.6 | 9.8 | 1.2 | 0.3 | Ref 2 |
| Brassica oleracea (#3) | – | Leaf | 2011.11 | n.d. | n.d. | 4.3 | −8.0 | 3.4 | −1.2 | −0.8 | 6.3 | −5.5 | n.d. | 3.4 | 11.7 | 1.0 | 0.3 | Ref 2 |
| Daucus carota | – | Leaf | 2011.11 | −30.6 | 5.9 | 8.3 | −2.2 | 8.0 | 4.6 | 7.3 | 7.2 | n.d. | n.d. | 8.2 | 15.7 | 1.1 | 0.3 | Ref 3 |
| Castanea crenata (#1) | – | Leaf | 2008.11 | −30.0 | 0.7 | −2.1 | −12.7 | −0.3 | 0.5 | 1.6 | 4.6 | −4.2 | n.d. | 1.5 | 10.1 | 1.0 | 0.3 | Ref 2 |
| Castanea crenata (#2) | – | Leaf | 2010.11 | −29.3 | 1.8 | 0.7 | −9.8 | 0.2 | −2.4 | −0.7 | 6.2 | n.d. | n.d. | 2.9 | 8.8 | 1.3 | 0.3 | This study |
| Castanea crenata (#3) | – | Nut | 2008.11 | n.d. | n.d. | n.d. | −14.8 | n.d. | 0.1 | n.d. | 2.9 | −7.2 | n.d. | 0.8 | 8.3 | 1.1 | 0.3 | Ref 2 |
| Citrus unshiu | – | Leaf | 2011.11 | −30.6 | 4.9 | 6.4 | −6.8 | 4.7 | 3.1 | 3.0 | 8.9 | −0.6 | n.d. | 2.8 | 12.4 | 0.8 | 0.3 | Ref 3 |
| Cucurbita moschata | – | Leaf | 2012.8 | −28.3 | 4.3 | 3.9 | −8.6 | 1.0 | −0.6 | 0.1 | 1.0 | −14.5 | n.d. | 2.1 | 10.1 | 1.1 | 0.3 | This study |
| Diospyros kaki Thunberg | – | Leaf | 2012.6 | −29.3 | 2.8 | −1.2 | −4.4 | −1.2 | −2.2 | 0.0 | 0.0 | −3.3 | n.d. | 1.0 | 8.8 | 1.1 | 0.3 | This study |
| Prunus avium | – | Leaf | 2012.6 | −30.1 | 3.8 | 1.9 | −2.9 | 2.9 | 2.1 | 1.2 | 2.9 | −4.3 | n.d. | 3.2 | 11.6 | 1.0 | 0.3 | This study |
| Raphanus sativus | – | Leaf | 2011.11 | −29.8 | 4.0 | −3.6 | −8.4 | −2.9 | −3.8 | −4.3 | 3.3 | −4.9 | n.d. | −2.6 | 5.9 | 1.0 | 0.3 | Ref 3 |
| Solanum lycopersicum | – | Leaf | 2011.11 | −28.5 | 5.2 | 6.2 | −3.6 | 2.0 | 2.9 | 1.3 | 8.6 | −4.0 | n.d. | 2.0 | 10.3 | 1.0 | 0.3 | Ref 3 |
| Solanum melongena | – | Leaf | 2011.11 | −27.7 | 5.6 | 5.6 | −4.6 | 6.8 | 1.3 | −0.2 | 15.1 | 5.1 | n.d. | 7.2 | 17.0 | 0.8 | 0.4 | Ref 3 |
| Solanum tuberosum | – | Leaf | 2011.11 | −29.5 | −2.8 | −2.4 | −12.1 | −2.0 | −5.7 | −3.5 | −2.0 | n.d. | n.d. | −6.3 | 4.1 | 0.7 | 0.4 | Ref 3 |
| Aphid | ||||||||||||||||||
| Aphidoidea sp. | Adult | Whole | 2011.11 | −21.8 | 2.2 | 5.5 | 2.9 | 7.1 | 3.6 | 5.1 | 10.4 | 1.2 | n.d. | 8.1 | 8.9 | 2.0 | 0.2 | Ref 3 |
| Butterfly | ||||||||||||||||||
| Hestina assimilis | Adult | Whole | 2011.8 | n.d. | n.d. | 3.9 | 3.9 | 13.6 | 12.6 | 15.7 | 17.0 | 3.1 | 2.1 | 10.5 | 11.7 | 2.0 | 0.2 | This study |
| Papilio machaon (#1) | Adult | Whole | 2011.9 | −27.3 | 2.8 | 9.1 | 2.4 | 11.8 | 3.6 | 9.5 | 14.7 | 7.5 | 1.7 | 11.0 | 12.5 | 1.9 | 0.3 | This study |
| Papilio protenor | Adult | Leg | 2012.9 | −29.1 | 6.0 | 12.6 | 6.6 | 8.5 | 7.2 | 10.2 | 19.1 | 6.8 | n.d. | 13.6 | 14.7 | 2.0 | 0.2 | This study |
| Pieris rapae (#1) | Larva | Whole | 2008.11 | −29.6 | 1.9 | 6.6 | −0.4 | 8.2 | 7.0 | 8.9 | 16.3 | 3.7 | 1.6 | 13.0 | 13.4 | 2.0 | 0.2 | Ref 2 |
| Pieris rapae (#2) | Larva | Whole | 2008.11 | −26.9 | 1.9 | 5.3 | −2.7 | 7.4 | 6.5 | 8.5 | 14.3 | 2.3 | 1.0 | 14.6 | 13.6 | 2.2 | 0.2 | Ref 2 |
| Pieris rapae (#3) | Larva | Whole | 2011.11 | n.d. | n.d. | 9.1 | 0.1 | 9.6 | 2.7 | 7.0 | 13.1 | 1.6 | n.d. | 10.6 | 9.5 | 2.2 | 0.2 | This study |
| Pieris rapae (#4) | Larva | Whole | 2011.11 | n.d. | n.d. | 8.3 | 0.8 | 6.5 | 2.4 | 4.6 | 11.8 | 1.5 | n.d. | 9.9 | 11.9 | 1.8 | 0.3 | This study |
| Pieris rapae (#5) | Adult | Whole | 2011.5 | −30.5 | 1.6 | 7.7 | 5.1 | 6.8 | 4.0 | 11.2 | 12.2 | 0.9 | −7.5 | 4.8 | 5.3 | 2.0 | 0.2 | This study |
| Pieris rapae (#6) | Adult | Whole | 2011.5 | n.d. | n.d. | 5.2 | 3.1 | 8.6 | 6.4 | 10.5 | 12.7 | 1.0 | 0.1 | 6.7 | 6.4 | 2.1 | 0.2 | This study |
| Bee | ||||||||||||||||||
| Apis mellifera (#1) | Adult | Whole | 2009.8 | −26.8 | 1.6 | 4.8 | 6.5 | 6.4 | 0.9 | 3.2 | 15.0 | 2.8 | 1.4 | 8.0 | 9.1 | 2.0 | 0.2 | Ref 3 |
| Apis mellifera (#2) | Adult | Whole | 2009.8 | n.d. | n.d. | 4.1 | 3.4 | 3.2 | −0.2 | 0.2 | 10.1 | 0.9 | n.d. | 7.3 | 7.2 | 2.1 | 0.2 | Ref 3 |
| Apis mellifera (#3) | Adult | Whole | 2009.8 | n.d. | n.d. | 6.3 | 6.9 | 7.5 | 5.8 | 5.5 | 20.8 | n.d. | 3.0 | 11.7 | 11.6 | 2.1 | 0.2 | Ref 3 |
| Bombus diversus diversus (#1) | Adult | Whole | 2010.10 | −26.9 | 2.2 | 2.4 | 2.1 | 2.0 | −0.8 | 0.3 | 12.2 | n.d. | −0.7 | 6.9 | 5.7 | 2.3 | 0.2 | Ref 3 |
| Bombus diversus diversus (#2) | Adult | Whole | 2012.5 | n.d. | n.d. | 5.1 | 0.0 | 4.3 | 3.2 | 5.2 | 4.1 | −1.1 | n.d. | 6.7 | 6.7 | 2.1 | 0.2 | This study |
| Xylocopa appendiculata (#1) | Adult | Whole | 2009.8 | −25.0 | 5.1 | 9.8 | 10.3 | 11.1 | 11.5 | 7.8 | 19.1 | 6.0 | −1.1 | 12.3 | 12.6 | 2.1 | 0.2 | Ref 3 |
| Xylocopa appendiculata (#2) | Adult | Whole | 2012.4 | n.d. | n.d. | 6.3 | 4.2 | 9.4 | 5.0 | 1.0 | 17.2 | 4.2 | −1.9 | 8.8 | 8.8 | 2.1 | 0.2 | This study |
| Katydid | ||||||||||||||||||
| Gampsocleis mikado | Adult | Leg | 2012.9 | −26.2 | 2.0 | 5.5 | −0.3 | 5.0 | 3.0 | 9.7 | n.d. | n.d. | −2.7 | 8.8 | 4.9 | 2.6 | 0.2 | This study |
| Holochlora japonica | Adult | Whole | 2011.11 | −26.4 | 9.1 | 9.7 | 4.5 | 11.2 | 9.5 | 12.4 | 15.6 | 1.8 | 1.2 | 12.1 | 11.9 | 2.1 | 0.2 | This study |
| Paper wasp | ||||||||||||||||||
| Polistes japonicus japonicus (#1) | Egg | Whole | 2010.8 | −27.2 | 4.9 | 7.9 | −1.0 | 16.4 | 7.6 | 10.0 | 16.7 | −1.6 | 0.0 | 19.9 | 14.1 | 2.9 | 0.2 | Ref 3 |
| Polistes japonicus japonicus (#2) | Larva | Whole | 2010.8 | −29.7 | 1.6 | 4.2 | 2.1 | 15.7 | 2.1 | 5.1 | 14.0 | 0.0 | −1.5 | 16.8 | 9.9 | 3.0 | 0.2 | Ref 3 |
| Polistes japonicus japonicus (#3) | Larva | Whole | 2010.8 | −30.1 | 1.1 | 4.6 | 1.0 | 14.4 | 1.4 | 5.0 | 13.6 | −0.6 | −3.4 | 17.3 | 8.7 | 3.2 | 0.3 | Ref 3 |
| Polistes japonicus japonicus (#4) | Chrysalis | Whole | 2010.8 | −29.4 | 1.4 | 3.6 | 1.5 | 16.4 | 5.2 | 7.4 | 17.5 | −0.5 | n.d. | 17.3 | 10.2 | 3.0 | 0.2 | Ref 3 |
| Polistes japonicus japonicus (#5) | Chrysalis | Whole | 2010.8 | −28.6 | 2.6 | 8.8 | 2.2 | 15.6 | 3.7 | 5.2 | 15.9 | −0.5 | n.d. | 18.6 | 11.2 | 3.1 | 0.2 | Ref 3 |
| Polistes japonicus japonicus (#6) | Newly-emerged | Whole | 2010.8 | −28.8 | 2.7 | 9.0 | 1.2 | 14.0 | 8.1 | 8.9 | 17.0 | −4.1 | −0.4 | 17.7 | 12.0 | 2.9 | 0.2 | Ref 3 |
| Polistes jokahamae jokahamae (#1) | Egg | Whole | 2012.7 | −29.3 | 4.5 | 10.9 | 6.5 | 10.8 | 5.8 | 6.1 | 14.1 | n.d. | n.d. | 12.7 | 6.6 | 2.9 | 0.2 | This study |
| Polistes jokahamae jokahamae (#2) | Larva | Whole | 2012.7 | −28.7 | 2.4 | 14.3 | 0.9 | 10.7 | 8.7 | 10.3 | 12.0 | n.d. | n.d. | 13.7 | 5.6 | 3.2 | 0.2 | This study |
| Polistes jokahamae jokahamae (#3) | Adult | Whole | 2012.7 | −29.9 | 3.9 | 13.7 | 3.0 | 10.5 | 10.4 | 8.9 | 8.8 | n.d. | n.d. | 13.1 | 5.4 | 3.1 | 0.2 | This study |
| Polistes mandarinus | Adult | Leg | 2012.11 | −23.7 | 5.5 | 7.6 | 8.0 | 9.8 | 8.8 | 18.9 | 16.1 | 7.3 | n.d. | 12.2 | 5.9 | 2.9 | 0.2 | This study |
| Polistes rothneyi iwatai (#1) | Egg | Whole | 2008.8 | −26.1 | 7.2 | 9.0 | 5.1 | 16.2 | 10.6 | 13.5 | 18.2 | 10.1 | n.d. | 22.5 | 13.2 | 3.3 | 0.3 | Ref 3 |
| Polistes rothneyi iwatai (#2) | Larva | Whole | 2008.8 | −27.4 | 4.6 | 7.4 | 2.7 | 14.3 | 8.2 | 12.3 | 16.3 | 4.3 | n.d. | 20.7 | 13.7 | 3.0 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#3) | Larva | Whole | 2008.8 | −27.2 | 5.5 | 8.0 | 0.9 | 16.0 | 9.8 | 13.6 | 17.9 | 1.2 | n.d. | 20.9 | 13.5 | 3.1 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#4) | Larva | Whole | 2008.8 | −27.2 | 5.2 | 8.3 | 5.5 | 15.3 | 9.0 | 13.9 | 18.4 | 5.8 | n.d. | 19.8 | 13.5 | 2.9 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#5) | Chrysalis | Whole | 2008.8 | −29.6 | 5.5 | 7.3 | 2.7 | 14.3 | 10.6 | 12.2 | 17.3 | −0.3 | n.d. | 20.3 | 12.9 | 3.1 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#6) | Chrysalis | Whole | 2008.8 | −28.6 | 5.5 | 6.0 | 3.3 | 15.1 | 10.0 | 14.2 | 18.1 | 0.8 | n.d. | 19.5 | 12.0 | 3.1 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#7) | Chrysalis | Whole | 2008.8 | −29.8 | 5.4 | 8.6 | 3.2 | 14.0 | 10.9 | 13.7 | 18.4 | 3.8 | n.d. | 20.5 | 13.6 | 3.0 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#8) | Chrysalis | Whole | 2008.8 | −28.1 | 4.9 | 9.5 | 8.5 | 15.9 | 12.0 | 15.3 | 16.5 | 1.6 | n.d. | 21.1 | 14.4 | 3.0 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#9) | Chrysalis | Whole | 2008.8 | −28.4 | 4.9 | 7.8 | 6.4 | 15.6 | 8.4 | 13.8 | 18.2 | 4.7 | n.d. | 19.6 | 13.5 | 2.9 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#10) | Chrysalis | Whole | 2008.8 | −28.2 | 4.9 | 6.4 | 10.0 | 16.7 | 12.5 | 15.6 | 16.7 | 0.5 | n.d. | 22.0 | 14.9 | 3.0 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#11) | Newly-emerged | Whole | 2008.8 | −28.4 | 2.4 | 5.5 | 1.2 | 11.7 | 9.7 | 10.9 | 14.2 | −0.6 | n.d. | 14.1 | 8.3 | 2.9 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#12) | Adult | Whole | 2008.8 | −26.8 | 5.5 | 12.2 | 8.4 | 9.0 | 5.6 | 5.6 | n.d. | 3.0 | 5.7 | 11.3 | 6.2 | 2.8 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#13) | Adult | Whole | 2009.8 | n.d. | n.d. | 6.1 | 5.5 | 8.8 | 7.5 | 11.0 | 12.6 | 4.0 | −1.8 | 14.3 | 6.1 | 3.2 | 0.2 | Ref 3 |
| Polistes rothneyi iwatai (#14) | Adult | Whole | 2009.8 | n.d. | n.d. | 5.9 | 4.5 | 8.6 | 5.6 | 8.6 | 13.6 | 3.0 | 2.7 | 15.9 | 8.4 | 3.1 | 0.2 | Ref 3 |
| Parapolybia indica (#1) | Larva | Whole | 2010.8 | −27.7 | 3.5 | 6.5 | 4.9 | 9.5 | 6.6 | 8.2 | 15.9 | 3.5 | n.d. | 14.9 | 9.1 | 2.9 | 0.2 | Ref 3 |
| Parapolybia indica (#2) | Larva | Whole | 2010.8 | −28.5 | 2.5 | 7.6 | 7.5 | 11.6 | 3.5 | 6.3 | 15.7 | 2.8 | n.d. | 13.8 | 6.3 | 3.1 | 0.2 | Ref 3 |
| Parapolybia indica (#3) | Larva | Whole | 2010.8 | −28.6 | 1.3 | 7.6 | 8.6 | 10.5 | 4.5 | 5.5 | 14.0 | 2.5 | n.d. | 12.0 | 5.1 | 3.0 | 0.2 | Ref 3 |
| Parapolybia indica (#4) | Chrysalis | Whole | 2010.8 | −28.4 | 3.7 | 8.0 | 8.4 | 11.1 | 6.0 | 9.6 | 16.2 | 2.6 | n.d. | 16.8 | 11.2 | 2.8 | 0.2 | Ref 3 |
| Parapolybia indica (#5) | Chrysalis | Whole | 2010.8 | −28.3 | 4.0 | 8.2 | 7.9 | 12.2 | 4.7 | 6.7 | 15.9 | 1.3 | n.d. | 12.7 | 4.8 | 3.1 | 0.2 | Ref 3 |
| Parapolybia indica (#6) | Chrysalis | Whole | 2010.8 | −27.6 | 3.3 | 7.6 | 5.8 | 11.4 | 5.9 | 7.2 | 13.9 | 3.4 | n.d. | 9.6 | 3.0 | 3.0 | 0.2 | Ref 3 |
| Parapolybia indica (#7) | Chrysalis | Whole | 2010.8 | −28.7 | 1.4 | 6.9 | 5.6 | 10.0 | 6.9 | 7.2 | 19.5 | 7.7 | n.d. | 10.0 | 4.4 | 2.8 | 0.2 | Ref 3 |
| Parapolybia indica (#8) | Newly-emerged | Whole | 2010.8 | −27.4 | 4.9 | 6.0 | 2.7 | 9.8 | 9.5 | 9.1 | 15.5 | 2.6 | n.d. | 14.9 | 8.2 | 3.0 | 0.2 | Ref 3 |
| Parapolybia indica (#9) | Adult | Whole | 2010.8 | −27.5 | 4.3 | 7.8 | 6.2 | 10.0 | 6.9 | 8.4 | 15.9 | 5.4 | n.d. | 15.6 | 8.8 | 3.0 | 0.2 | Ref 3 |
| Ant | ||||||||||||||||||
| Formica japonica | Adult | Whole | 2010.8 | n.d. | n.d. | 12.7 | 11.0 | 18.8 | 8.3 | 11.0 | 12.4 | n.d. | n.d. | 12.2 | 5.4 | 3.0 | 0.2 | This study |
| Ladybird beetle | ||||||||||||||||||
| Coccinella septempunctata (#1) | Adult | Whole | 2011.5 | −27.6 | 9.1 | 8.0 | 5.9 | 7.5 | 5.8 | 8.1 | 16.8 | 3.3 | 0.2 | 10.1 | 3.5 | 3.0 | 0.2 | This study |
| Coccinella septempunctata (#2) | Adult | Whole | 2011.10 | n.d. | n.d. | 8.5 | 6.6 | 7.7 | 6.8 | 8.4 | 19.0 | n.d. | n.d. | 10.8 | 3.6 | 3.0 | 0.2 | This study |
| Harmonia axyridis (#1) | Larva | Whole | 2011.11 | −26.5 | 6.4 | 10.8 | 8.4 | 9.4 | 4.9 | 9.1 | 18.7 | 3.7 | n.d. | 16.5 | 9.0 | 3.1 | 0.2 | Ref 3 |
| Harmonia axyridis (#2) | Chrysalis | Whole | 2011.11 | −29.0 | 4.7 | 10.2 | 7.9 | 10.1 | 5.0 | 7.8 | 15.0 | 3.7 | n.d. | 16.6 | 9.4 | 3.1 | 0.2 | Ref 3 |
| Harmonia axyridis (#3) | Adult | Whole | 2011.11 | −28.6 | 7.4 | 10.9 | 11.7 | 9.8 | 7.4 | 8.8 | 23.0 | 6.6 | n.d. | 14.2 | 6.9 | 3.1 | 0.2 | Ref 3 |
| Harmonia axyridis (#4) | Adult | Whole | 2012.4 | n.d. | n.d. | 10.1 | 6.5 | 2.3 | 3.6 | 5.1 | 18.9 | −9.1 | n.d. | 10.3 | 4.0 | 2.9 | 0.2 | This study |
| Harmonia axyridis (#5) | Adult | Whole | 2012.4 | n.d. | n.d. | 10.9 | 6.7 | 5.4 | 4.4 | 4.4 | 19.1 | 1.4 | n.d. | 10.6 | 3.1 | 3.1 | 0.2 | This study |
| Harmonia axyridis (#6) | Adult | Whole | 2012.4 | n.d. | n.d. | 7.7 | 5.3 | 3.9 | 4.3 | 6.2 | 5.9 | −8.4 | 10.5 | 2.8 | 3.1 | 0.2 | This study | |
| Harmonia axyridis (#6) | Adult | Whole | 2012.4 | n.d. | n.d. | 13.0 | 10.2 | 4.2 | −0.1 | −0.9 | 19.1 | n.d. | n.d. | 11.9 | 4.2 | 3.1 | 0.2 | This study |
| Illeis koebelei (#1) | Adult | Whole | 2012.10 | n.d. | n.d. | 10.8 | 6.7 | 5.6 | 5.6 | 8.6 | 19.1 | 3.0 | n.d. | 12.7 | 5.5 | 3.1 | 0.2 | This study |
| Illeis koebelei (#2) | Adult | Whole | 2012.10 | n.d. | n.d. | 10.4 | 6.5 | 9.3 | 6.5 | 8.8 | 19.4 | −1.1 | n.d. | 12.0 | 5.5 | 3.0 | 0.2 | This study |
| Illeis koebelei (#3) | Adult | Whole | 2012.11 | n.d. | n.d. | 11.2 | 5.3 | 8.9 | 7.4 | 14.0 | 20.3 | n.d. | n.d. | 13.6 | 7.1 | 3.0 | 0.2 | This study |
| Illeis koebelei (#4) | Adult | Whole | 2012.11 | n.d. | n.d. | 14.8 | 9.7 | 13.9 | 9.2 | 14.5 | n.d. | 6.7 | 1.5 | 17.0 | 8.5 | 3.2 | 0.3 | This study |
| Illeis koebelei (#5) | Adult | Whole | 2012.11 | n.d. | n.d. | 12.6 | 6.0 | 12.2 | 6.3 | 10.4 | n.d. | n.d. | n.d. | 14.1 | 6.8 | 3.1 | 0.2 | This study |
| Menochilus sexmaculatus (#1) | Adult | Whole | 2012.4 | n.d. | n.d. | 8.4 | 2.8 | 5.5 | 8.0 | 9.4 | n.d. | n.d. | n.d. | 8.8 | 1.6 | 3.1 | 0.2 | This study |
| Menochilus sexmaculatus (#2) | Adult | Whole | 2012.4 | n.d. | n.d. | 10.3 | 6.3 | 10.3 | 11.4 | 10.3 | n.d. | n.d. | n.d. | 12.1 | 4.1 | 3.2 | 0.2 | This study |
| Mantid | ||||||||||||||||||
| Tenodera aridifolia | Adult | Wing | 2012.9 | n.d. | n.d. | 9.5 | 7.0 | 11.0 | 8.9 | 11.0 | 20.7 | n.d. | n.d. | 14.4 | 5.9 | 3.2 | 0.3 | This study |
| Hornet | ||||||||||||||||||
| Vespa analis fabriciusi (#1) | Egg | Whole | 2011.6 | −25.7 | 6.8 | 16.9 | 13.3 | 20.0 | 13.7 | 18.5 | 23.5 | 7.7 | 0.5 | 20.3 | 7.4 | 3.8 | 0.3 | This study |
| Vespa analis fabriciusi (#2) | Larva | Whole | 2011.6 | −25.6 | 4.9 | 13.4 | 13.0 | 18.0 | 11.7 | 17.2 | 18.5 | 7.1 | 0.6 | 18.4 | 7.4 | 3.6 | 0.3 | This study |
| Vespa analis fabriciusi (#3) | Larva | Whole | 2011.6 | −25.6 | 4.5 | 12.9 | 14.0 | 16.9 | 11.4 | 16.2 | 18.5 | 6.7 | 0.4 | 18.8 | 7.1 | 3.6 | 0.3 | This study |
| Vespa analis fabriciusi (#4) | Larva | Whole | 2011.6 | −25.7 | 3.7 | 11.4 | 13.7 | 15.7 | 7.8 | 14.3 | 16.5 | 4.9 | −1.3 | 14.5 | 3.6 | 3.5 | 0.3 | This study |
| Vespa analis fabriciusi (#5) | Chrysalis | Whole | 2011.6 | −26.6 | 4.7 | 13.4 | 14.6 | 18.6 | 9.9 | 16.6 | 18.8 | 6.0 | n.d. | 17.6 | 6.1 | 3.6 | 0.3 | This study |
| Vespa analis fabriciusi (#6) | Chrysalis | Whole | 2011.6 | −26.1 | 5.0 | 14.3 | 13.4 | 17.7 | 12.1 | 19.1 | 20.0 | 8.3 | n.d. | 16.6 | 6.0 | 3.5 | 0.3 | This study |
| Vespa analis fabriciusi (#7) | Newly-emerged | Whole | 2011.6 | −26.2 | 5.0 | 15.4 | 15.7 | 19.9 | 14.5 | 17.0 | 23.5 | 14.0 | −0.3 | 17.7 | 6.3 | 3.6 | 0.3 | This study |
| Vespa ducalis pulchra (#1) | Adult | Whole | 2009.8 | −25.8 | 5.4 | 7.6 | 5.4 | 19.9 | 10.7 | 13.2 | 19.1 | 4.5 | n.d. | 21.4 | 7.7 | 3.9 | 0.3 | Ref 3 |
| Vespa ducalis pulchra (#2) | Adult | Whole | 2009.8 | n.d. | n.d. | 10.7 | 9.5 | 17.2 | 10.1 | 13.2 | 20.6 | 6.8 | n.d. | 23.5 | 8.8 | 4.0 | 0.3 | Ref 3 |
| Vespa ducalis pulchra (#3) | Adult | Whole | 2009.8 | n.d. | n.d. | 10.0 | 4.5 | 14.8 | 11.0 | 15.5 | 16.1 | 3.0 | n.d. | 22.6 | 7.4 | 4.1 | 0.3 | Ref 3 |
| Vespa mandarinia japonica (#1) | Adult | Whole | 2010.10 | −26.8 | 4.5 | 13.2 | −0.2 | 16.8 | 6.6 | 8.5 | 15.3 | 0.6 | 1.0 | 18.5 | 8.0 | 3.5 | 0.3 | Ref 3 |
| Vespa mandarinia japonica (#2) | Adult | Whole | 2012.7 | n.d. | n.d. | 19.0 | 1.1 | 22.2 | 13.8 | 10.5 | 24.3 | 2.1 | −0.1 | 20.6 | 11.6 | 3.3 | 0.3 | This study |
| Vespa mandarinia japonica (#3) | Adult | Whole | 2012.7 | n.d. | n.d. | 23.4 | 6.5 | 22.7 | 14.7 | 11.5 | 30.0 | 3.2 | 5.3 | 25.3 | 13.6 | 3.6 | 0.3 | This study |
| Vespa simillima xanthoptera | Adult | Whole | 2009.8 | −25.6 | 4.9 | 12.0 | 7.0 | 12.2 | 7.2 | 10.2 | 18.3 | 3.6 | n.d. | 20.1 | 9.4 | 3.5 | 0.3 | Ref 3 |
| Vespula flaviceps lewisii | Adult | Whole | 2010.10 | −24.6 | 5.6 | 12.3 | 5.6 | 16.6 | 7.2 | 10.0 | n.d. | 5.9 | n.d. | 20.0 | 9.7 | 3.5 | 0.3 | Ref 3 |
n.d.: Not determined.
The δ15N value was determined by single analysis for each sample.
TPGlu/Phe = (δ15NGlu − δ15NPhe + 8.4)/7.6 + 1.
Propagation error on the TPGlu/Phe value based on 1σ on the δ15N measurement of amino acids in this study and 1σ on the β and TDF values reported in Chikaraishi et al. 2010.
Conflict of Interest
None declared.
References
- Aita MN, Tadokoro K, Ogawa NO, Hyodo F, Ishii R, Smith SL, et al. Linear relationship between carbon and nitrogen isotope ratios along simple food chains in marine environments. J. Plankton Res. 2011;33:1629–1642. [Google Scholar]
- Bearhop S, Adams CE, Waldron S, Fuller RA, Macleod H. Determining trophic niches width: a novel approach using stable isotope analysis. J. Anim. Ecol. 2004;73:1007–1012. [Google Scholar]
- Bloonfield AL, Elsdon TS, Walther BD, Gier EJ, Gilanders BM. Temperature and diet affect carbon and nitrogen isotopes of fish muscle: can amino acid nitrogen isotopes explain effects? J. Exp. Mar. Biol. Ecol. 2011;399:48–59. [Google Scholar]
- Bruno JF, O'Connor MJ. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Lett. 2005;8:1048–1056. [Google Scholar]
- Cabana G, Rasmussen JB. Comparison of aquatic food chains using nitrogen isotopes. Proc. Natl Acad. Sci. 1996;93:10844–10847. doi: 10.1073/pnas.93.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JM, Leibold MA. Ecological niches. Chicago, IL: Univ. of Chicago Press; 2003. [Google Scholar]
- Chikaraishi Y, Kashiyama Y, Ogawa NO, Kitazato H, Ohkouchi N. Biosynthetic and metabolic controls of nitrogen isotopic composition of amino acids in marine macroalgae and gastropods: implications for aquatic food web studies. Mar. Ecol. Prog. Ser. 2007;342:85–90. [Google Scholar]
- Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, et al. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods. 2009;7:740–750. [Google Scholar]
- Chikaraishi Y, Ogawa NO. Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids. In: Ohkouchi N, Tayasu I, Koba K, Ohkouchi N, editors. Earth, life, and isotopes. Kyoto, Japan: Kyoto Univ. Press; 2010. pp. 37–51. [Google Scholar]
- Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N. 15N/14N ratios of amino acids as a tool for studying terrestrial food webs: a case study of terrestrial insects (bees, wasps, and hornets) Ecol. Res. 2011;26:835–844. [Google Scholar]
- Coll M, Guershon M. Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu. Rev. Entomol. 2002;47:267–297. doi: 10.1146/annurev.ento.47.091201.145209. [DOI] [PubMed] [Google Scholar]
- Corr LT, Berstan R, Evershed RP. Development of N-acetyl methyl ester derivatives for the determination of δ13C values of amino acids using gas chromatography-combustion-isotope ratio mass spectrometry. Anal. Chem. 2007;79:9082–9090. doi: 10.1021/ac071223b. [DOI] [PubMed] [Google Scholar]
- Dale JJ, Wallsgrove NJ, Popp BN, Holland KN. Nursery habitat use and foraging ecology of the brown stingray Dasyatis lata determined from stomach contents, bulk and amino acid stable isotopes. Mar. Ecol. Prog. Ser. 2011;433:221–236. [Google Scholar]
- Darnell RM. Trophic spectrum of an estuarine community based on studies of Lake Pontchartrain, Louisiana. Ecology. 1961;42:553–568. [Google Scholar]
- Décima M, Landry MR, Popp BN. Environmental perturbation effects on baseline δ15N values and zooplankton trophic flexibility in the Southern California Current Ecosystem. Limnol. Oceanogr. 2013;58:624–634. [Google Scholar]
- DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Coshmochimica Acta. 1978;42:495–506. [Google Scholar]
- DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta. 1981;45:341–351. [Google Scholar]
- Duffy JE, Cardinales BJ, France KE, McIntyre PB, Thébault E. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Dunn PJH, Honch NV, Evershed RP. Comparison of liquid chromatography–isotope ratio mass spectrometry (LC/IRMS) and gas chromatography–combustion–isotope ratio mass spectrometry (GC/C/IRMS) for the determination of collagen amino acid δ13C values for palaeodietary and palaeoecological reconstruction. Rapid Commun. Mass Spectrom. 2011;25:295–3011. doi: 10.1002/rcm.5174. [DOI] [PubMed] [Google Scholar]
- ElEla SA, ElSayed W, Nakamura K. Mandibular structure, gut contents analysis and feeding group of orthopteran species collected from different habitats of Satoyama area within Kanazawa City, Japan. J. Threat. Taxa. 2010;2:849–857. [Google Scholar]
- Finke DL, Denno RF. Predator diversity and the functioning of ecosystems: The role of intraguild predation in dampening trophic cascades. Ecol. Lett. 2005;8:1299–1306. [Google Scholar]
- Germain LR, Koch PL, Harvey J, McCarthy MD. Nitrogen isotope fractionation in amino acids from harbor seals: implications for compound-specific trophic position calculations. Mar. Ecol. Prog. Ser. 2013;482:265–277. [Google Scholar]
- Griffin JN, de la Haye KL, Hawkins SJ, Thompson RC, Jenkins SR. Predator diversity and ecosystem functioning: density modifies the effect of resource partitioning. Ecology. 2008;89:298–305. doi: 10.1890/07-1220.1. [DOI] [PubMed] [Google Scholar]
- Hannides CCS, Popp BN, Landry MR, Graham BS. Quantification of zooplankton trophic position in the North Pacific Subtropical Gytr using stable nitrogen isotopes. Limnol. Oceanogr. 2009;54:50–61. [Google Scholar]
- Hare PE, Fogel ML, Mitchell TW, Jr, Stafford AD, Hoering TC. The isotopic composition of carbon and nitrogen in individual amino acids Isolated from modern and fossil proteins. J. Archaeol. Sci. 1991;18:277–292. [Google Scholar]
- Howland MR, Corr LT, Young SMM, Jones V, Jim S, van der Merwe NJ, et al. Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. Int. J. Osteoarchaeol. 2003;13:54–65. [Google Scholar]
- Jaksić FM, Delibes M. A comparative analysis of food-niche relationships and trophic guild structure in two assemblages of vertebrate predators differing in species richness: causes, correlations, and consequences. Oecologia. 1987;71:461–472. doi: 10.1007/BF00378722. [DOI] [PubMed] [Google Scholar]
- Larsen T, Taylor DL, Leigh MB, O'Brien DM. Stable isotope fingerprinting: a novel method for identifying plant, fungal, or bacterial origins of amino acids. Ecology. 2009;90:3526–3535. doi: 10.1890/08-1695.1. [DOI] [PubMed] [Google Scholar]
- Larsen T, Ventura M, Andersen N, O'Brine DM, Piatkowski U, McCarthy MD. Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS ONE. 2013;8:e73441. doi: 10.1371/journal.pone.0073441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain A, Graham B, Ménard F, Popp BN, Bouillon S, van Breugel P, et al. Nitrogen and carbon isotope values of individual amino acids: a tool to study foraging ecology of penguins in the Southern Ocean. Mar. Ecol. Prog. Ser. 2009;391:293–306. [Google Scholar]
- Maeda T, Hirose E, Chikaraishi Y, Kawato M, Takishita K, Yoshida T, et al. Algivore or phototroph? Plakobranchus ocellatus (Gastropoda) continuously acquires kleptoplasts and nutrition from multiple algal species in nature. PLoS ONE. 2012;7:e42024. doi: 10.1371/journal.pone.0042024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MD, Benner R, Lee C, Fogel ML. Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim. Cosmochim. Acta. 2007;71:4727–4744. [Google Scholar]
- McCarthy MD, Lehman J, Kudela R. Compound-specific amino acid δ15N patterns in marine algae: Tracer potential for cyanobacterial vs. eukaryotic organic nitrogen sources in the ocean. Geochim. Cosmochim. Acta. 2013;103:104–120. [Google Scholar]
- McClelland JW, Montoya JP. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology. 2002;83:2173–2180. [Google Scholar]
- McClelland JW, Holl CM, Montoya JP. Relating low δ15N values of zooplankton to N2-fixation in the tropical North Atlantic: insights provided by stable isotope ratios of amino acids. Deep-Sea Res. I. 2003;50:849–863. [Google Scholar]
- McMahon KW, Fogel ML, Eisdon TS, Thorrld SR. Carbon isotope fractionation of amino acids in fish muscle reflects biosynthesis and isotopic routing from dietary protein. J. Anim. Ecol. 2010;79:1132–1141. doi: 10.1111/j.1365-2656.2010.01722.x. [DOI] [PubMed] [Google Scholar]
- McMahon KW, Berumen ML, Thorrold SR. Linking habitat mosaics and connectivity in a coral reef seascape. Proc. Natl Acad. Sci. 2012;109:15372–15376. doi: 10.1073/pnas.1206378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Chikaraishi Y, Ogawa NO, Tamade Y, Tsukamoto K, Ohkouchi N. A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol. Lett. 2012;9:20120826. doi: 10.1098/rsbl.2012.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidences and the relation between δ15N and animal age. Geochim. Cosmochim. Acta. 1984;48:1135–1140. [Google Scholar]
- Naito YI, Honch NV, Chikaraishi Y, Ohkouchi N, Yoneda M. Quantitative evaluation of marine protein contribution in ancient diets based on nitrogen isotope ratios of individual amino acids in bone collagen: an investigation at the Kitakogane Jomon site. Am. J. Phys. Anthropol. 2010;143:31–40. doi: 10.1002/ajpa.21287. [DOI] [PubMed] [Google Scholar]
- Naito YI, Chikaraishi Y, Ohkouchi N, Yoneda M. Evaluation of carnivory in inland Jomon hunter–gatherers based on nitrogen isotopic compositions of individual amino acids in bone collagen. J. Archaeol. Sci. 2013;40:2913–2923. [Google Scholar]
- O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: Amino acid turnover and allocation to reproduction in Lepidoptera. Proc. Natl Acad. Sci. 2002;99:4413–4418. doi: 10.1073/pnas.072346699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa NO, Chikaraishi Y, Ohkouchi N. Trophic position estimates of formalin-fixed samples with nitrogen isotopic compositions of amino acids: an application to gobiid fish (Isaza) in Lake Biwa, Japan. Ecol. Res. 2013;28:697–702. [Google Scholar]
- Pimm SL. The balance of nature? Chicago, IL: Univ. of Chicago Press; 1991. [Google Scholar]
- Polis GA. Complex trophic interactions in deserts: an empirical critique of food-web theory. Am. Nat. 1991;138:123–155. [Google Scholar]
- Polis GA, Strong DR. Food web complexity and community dynamics. Am. Nat. 1996;147:813–846. [Google Scholar]
- Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott M, López-Ibarra G, et al. Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson TE, Siegwolf RTW, editors. Stable isotopes as indicators of ecological change. San Diego, USA: Academic Press; 2007. pp. 173–190. [Google Scholar]
- Post DM. Using stable isotopes to estimate trophic position: models. Methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- Power ME, Matthews WJ, Stewart AJ. Grazing minnows, piscivorous bass and stream algae: dynamics of a strong interaction. Ecology. 1985;66:1448–1456. [Google Scholar]
- Rosenheim JA. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 1998;43:421–447. doi: 10.1146/annurev.ento.43.1.421. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cooley RI, Balance LT, McCarthy MD. Range expansion of the jumbo squid in the NE Pacific: δ15N decrypts multiple origins, migration and habitat use. PLoS ONE. 2013;8:e59651. doi: 10.1371/journal.pone.0059651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood OA, Iehmann MF, Schuber CJ, Scott DB, McCarthy MD. Nutrient regime shift in the western North Atlantic indicated by compound-specific δ15N of deep-sea gorgonian corals. Proc. Natl Acad. Sci. 2011;108:1011–1015. doi: 10.1073/pnas.1004904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Englund G, Wooster D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. [DOI] [PubMed] [Google Scholar]
- Smith CI, Fuller BT, Choy K, Richards MP. A three-phase liquid chromatographic method for δ13C analysis of amino acids from biological protein hydrolysates using liquid chromatography–isotope ratio mass spectrometry. Anal. Biochem. 2009;390:165–172. doi: 10.1016/j.ab.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Steffan SA, Chikaraishi Y, Horton DR, Ohkouchi N, Singleton ME, Miliczky E, et al. Trophic hierarchies illuminated via amino acid isotopic analysis. PLoS ONE. 2013;9:e76152. doi: 10.1371/journal.pone.0076152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styring AK, Sealy JC, Evershed RP. Resolving the bulk δ15N values of ancient human and animal bone collagen via compound-specific nitrogen isotope analysis of constituent amino acids. Geochmica et Cosmochimica Acta. 2010;74:241–251. [Google Scholar]
- Styring AK, Kuhl A, Knowles TDJ, Fraser RA, Bogaard A, Evershed RP. Practical considerations in the determination of compound-specific amino acid δ15N values in animal and plant tissues by gas chromatography-combustion-isotope ratio mass spectrometry, following derivatisation to their N-acetylisopropyl esters. Rapid Commun. Mass Spectrom. 2012;26:2328–2334. doi: 10.1002/rcm.6322. [DOI] [PubMed] [Google Scholar]
- Takamizawa K. The Japanese social wasps and bees. Nagano, Japan: The Shinano Mainichi Shinbun; 2005. [Google Scholar]
- Thompson RM, Hemberg M, Starzomski BM, Shurin JB. Trophic levels and trophic tangles: the prevalence of omnivory in real food webs. Ecology. 2007;88:612–617. doi: 10.1890/05-1454. [DOI] [PubMed] [Google Scholar]
- Vander Zanden MJ, Rasmussen JB. Primary consumer Δ13C and Δ15N and the trophic position of aquatic consumers. Ecology. 1999;80:1395–1404. [Google Scholar]
- Vander Zanden MJ, Rasmussen JB. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol. Oceanogr. 2001;46:2061–2066. [Google Scholar]
- Vander Zanden MJ, Cabana G, Rasmussen JB. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N) and literature dietary data. Can. J. Fish. Aquat. Sci. 1997;54:1142–1158. [Google Scholar]
- Vander Zanden HB, Arthur KE, Bolten AB, Popp BN, Lagueux CJ, Harrison E, et al. Trophic ecology of a green turtle breeding population. Mar. Ecol. Prog. Ser. 2013;476:237–249. [Google Scholar]
- Williams RJ, Martinez ND. Trophic levels in complex food webs: theory and data. Am. Nat. 2004;163:458–468. doi: 10.1086/381964. [DOI] [PubMed] [Google Scholar]
- Yoshii K, Melnik NG, Timoshkin OA, Bondarenko NA, Anoshko PN, Yoshida T, et al. Stable isotope analyses of the pelagic food web in Lake Baikal. Limnol. Oceanogr. 1999;44:502–511. [Google Scholar]