Abstract
The birth of new neurons in the walls of the adult brain lateral ventricles has captured the attention of many neuroscientists for over two decades, yielding key insights into the identity and regulation of neural stem cells (NSCs). In the adult ventricular-subventricular zone (V-SVZ), NSCs are a specialized form of astrocyte that generates several types of neurons for the olfactory bulb. Here we discuss recent findings regarding the unique organization of the V-SVZ NSCs niche, the multiple regulatory controls of neuronal production, the distinct regional identities of adult NSCs, and the epigenetic mechanisms that maintain adult neurogenesis. Understanding how V-SVZ NSCs establish and maintain lifelong neurogenesis continues to provide surprising insights into the cellular and molecular regulation of neural development.
Keywords: neurogenesis, neural stem cell, niche, regional identity, neurotransmitter, epigenetics, chromatin
Since the discovery of adult brain neurogenesis [1, 2], a finding that overturned the long-held notion “of no new neurons” in the adult central nervous system, exploration into the cellular and molecular regulation of adult neural stem cells (NSCs) has continued to generate fundamental insights into both neural developmental and adult brain function. One of the most interesting questions unique to the study of adult neurogenesis is how adult NSCs – which are derived from progenitor cells in the embryo – retain both their neurogenic capacity and regional specificity for long periods of time.
In the brain of many mammals, a large population of NSCs is present within an epithelium called the ventricular-subventricular zone (V-SVZ) that lines the walls of the lateral ventricle [3, 4]. In rodents, V-SVZ NSCs generate large numbers of neurons that migrate very rapidly along the rostral migratory stream (RMS) into the olfactory bulb (OB), were they differentiate into multiple types of local circuit interneurons. The OB function and structure relies on this constant inflow of new neurons that contribute to neural plasticity of olfactory information processing [5, 6].
The identification of a subpopulation of astroglial cells (B1 cells) as the V-SVZ NSCs [7] provided some of the first clues about the glial nature of NSCs, a concept that has been generalized to embryonic development [8]. In both the embryo and adult brain, NSCs are a specialized form of glia that reside in neurogenic niches [9]. However, in contrast to the embryonic brain – wherein neural precursors are inherently transient, continually changing their developmental potential and location over time – adult V-SVZ NSCs are more stable and harbored in a well-defined niche that includes ependymal cells, mature vasculature and axonal terminals. This unique niche provides multiple regulatory controls for the production of neurons within the fully assembled adult brain. Importantly, recent findings indicate that NSCs within the V-SVZ have distinct regional identities related to their embryonic origin. Thus, the adult NSC populations appear to “remember” positional cues that pattern the developing brain. Alongside advancement in the field of epigenetics – the study of heritable patterns of gene expression that do not involve changes to the DNA sequence – studies of the V-SVZ have revealed how epigenetic factors such as chromatin-modifying factors play critical roles in adult brain neurogenesis.
In this review, we focus on selected recent findings that illustrate how the study of the V-SVZ and OB neurogenesis offer unique advantages for discovery of developmental processes and molecular mechanisms that have long been thought to be restricted to embryonic development.
Type B1 cells: a “displaced” form of radial glia?
Consistent with their astrocytic morphology and ultrastructure, B1 cells express glial markers such as the glial-fibrillary acidic protein (GFAP), glutamate aspartate transporter (GLAST), and brain lipid binding protein (BLBP). Recent work indicates that B1 cells can exist in either a quiescent or activated state [10, 11]. Interestingly, quiescent B1 cells do not appear to express Nestin, an intermediate filament protein that has long been considered to be a marker of NSCs. Activated B1 cells express Nestin and generate transit-amplifying precursors (C cells) that divide symmetrically approximately three times before becoming migratory neuroblasts (A cells), which divide one or two more times while en route to the OB [12]. Type A cells migrate within a network of interconnecting paths that coalesce at the anterior ventricle, forming the rostral migratory stream (RMS) [13], which carries the neuroblasts into the OB where they then migrate radially and differentiate into interneurons of several different types, as we later discuss.
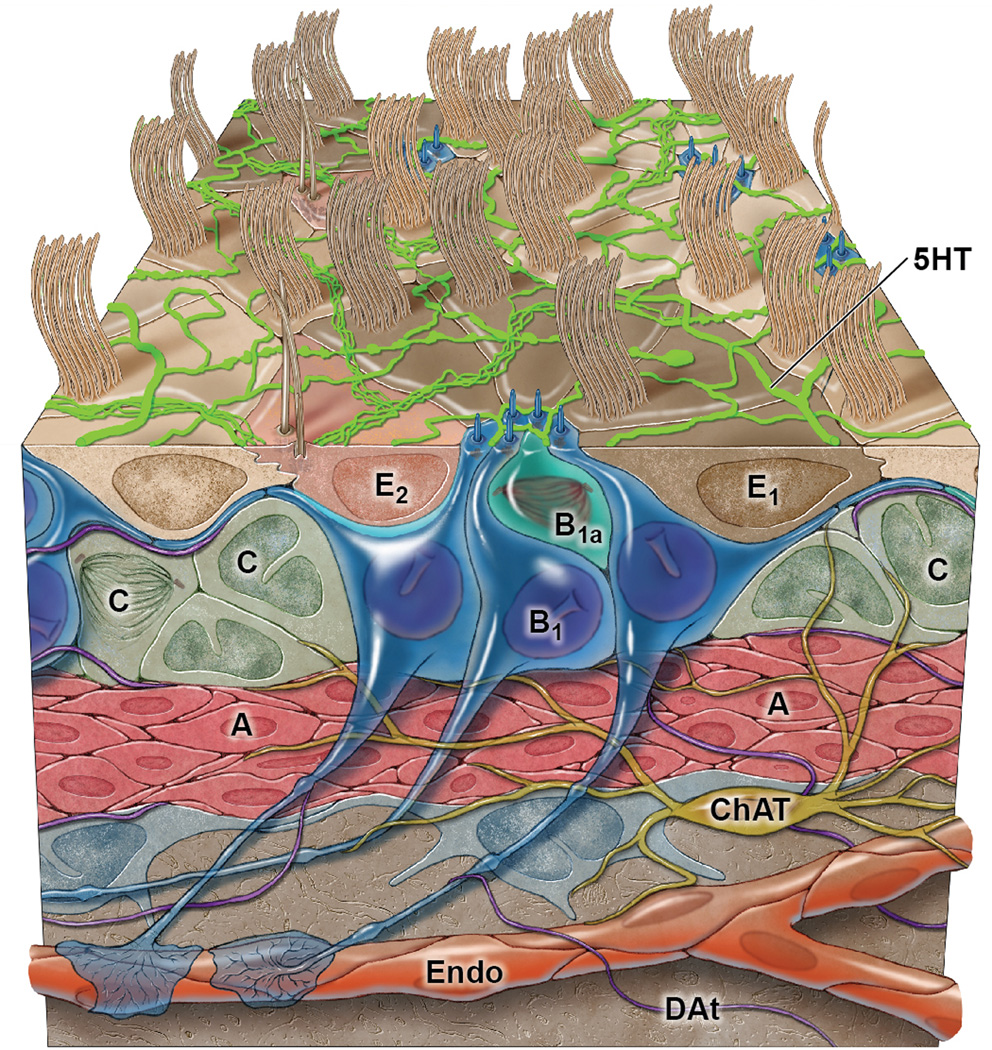
B1 cells retain epithelial features similar to those of their predecessors [14] the radial glia, which are the precursors to most neurons and mature glia in the embryo. B1 cells have apical processes that contact the ventricle and end-feet on blood vessels [3, 4]. This elongated structure allows B1 cells to bridge all compartments of the V-SVZ (Fig. 1). The V-SVZ can be subdivided into three domains based on the structure and spatial arrangement of B1 cells: Domain I (apical) contains the apical process of B1 cells and the ependymal layer; domain II (intermediate) contains the cell body of most type B1 cells, which are in contact with the type C and A cells; and domain III (basal) contains the B1 cell’s basal process with end-feet upon blood vessels. These subdomains likely play unique roles in type B1 cell regulation, perhaps by providing NSCs with extrinsic signals that are distinct to each region.
Figure 1. Schematic of the V-SVZ organization.
B1 cells, V-SVZ NSCs (dark blue) give rise to activated B1 cells (B1a, light blue) that actively divide [10, 11]. Activated B1 cells generate the transit-amplifying C cells (green) that after 3 rounds of divisions give rise to A cells, the migrating neuroblasts [12]. Note that B1 cells contact the ventricle with an apical process. This adult VZ is also populated by ependymal cells, multiciliated cells that together with the apical endings of B1 cells from pinwheel structures on the surface [3]. Coursing along this ventricular surface is a rich network of serotonergic axons (5HT, bright green) [44]. The basal process of B1 cells has endings on blood vessels. Choline acetyltransferase (ChAT) -positive neurons found in the region have endings in the SVZ (olive brown) [51]. Dopaminergic terminals (DAt, purple) are also observed in this region.
Prior to studies of the V-SVZ, the lateral ventricle ependyma was generally described as a layer of multiciliated epithelial cells forming a “barrier” between the brain parenchyma and the ventricle lumen, which contains cerebrospinal fluid (CSF). However, in domain I, B1 cells contact the ventricle with a thin cellular process that is interdigitated between ependymal cells [7, 15, 16]; when the surface of the ventricle is viewed en face, these thin apical endings are observed at the center of pinwheels formed by the large apical surfaces of the surrounding ependymal cells [3]. While ependymal cells bear many long, motile cilia, the apical surface of type B1 cells has a single, non-motile primary cilium.
Interestingly, primary cilium and ventricular contact are cellular features common to embryonic radial glial cells [9]. In early neural precursors, the primary cilium is required for SHH signaling [17]. In the V-SVZ, SHH-signaling regulates neurogenesis [18], and the CSF is known to contain a number of extrinsic signals including SHH, Wnts, retinoic acid (RA), bone morphogenetic proteins (BMPs), and insulin-like growth factor 2 (IGF-2) [19, 20]. IGF-2 in the CSF regulates the proliferation of type B1 cells [20], supporting the notion that the CSF provides extrinsic regulatory cues to the V-SVZ niche; however, it remains to be determined whether the ventricular contact and/or the primary cilium of B1 cells are required for the transduction of factors in the CSF.
The spatial and morphological similarities between B1 cells and embryonic radial glia and the direct lineage relationship between these populations of neural precursors, suggest that B1 cells “preserve” certain embryonic-like characteristics throughout adult life. The persistence of long-term neurogenesis likely involves both cell-intrinsic neurogenic “competence” of B1 cells and cell-extrinsic signals that are instructive or permissive for neuronal differentiation. During early embryonic development, morphogens pattern the brain, inducing radial glial cells to acquire regional identities. As we discuss below, B1 cells also maintain similar regional identities throughout adult life. This long-term maintenance of regional identity in NSC populations raises important questions about how such spatial information is maintained as the brain grows much larger.
The regional identity of V-SVZ NSCs
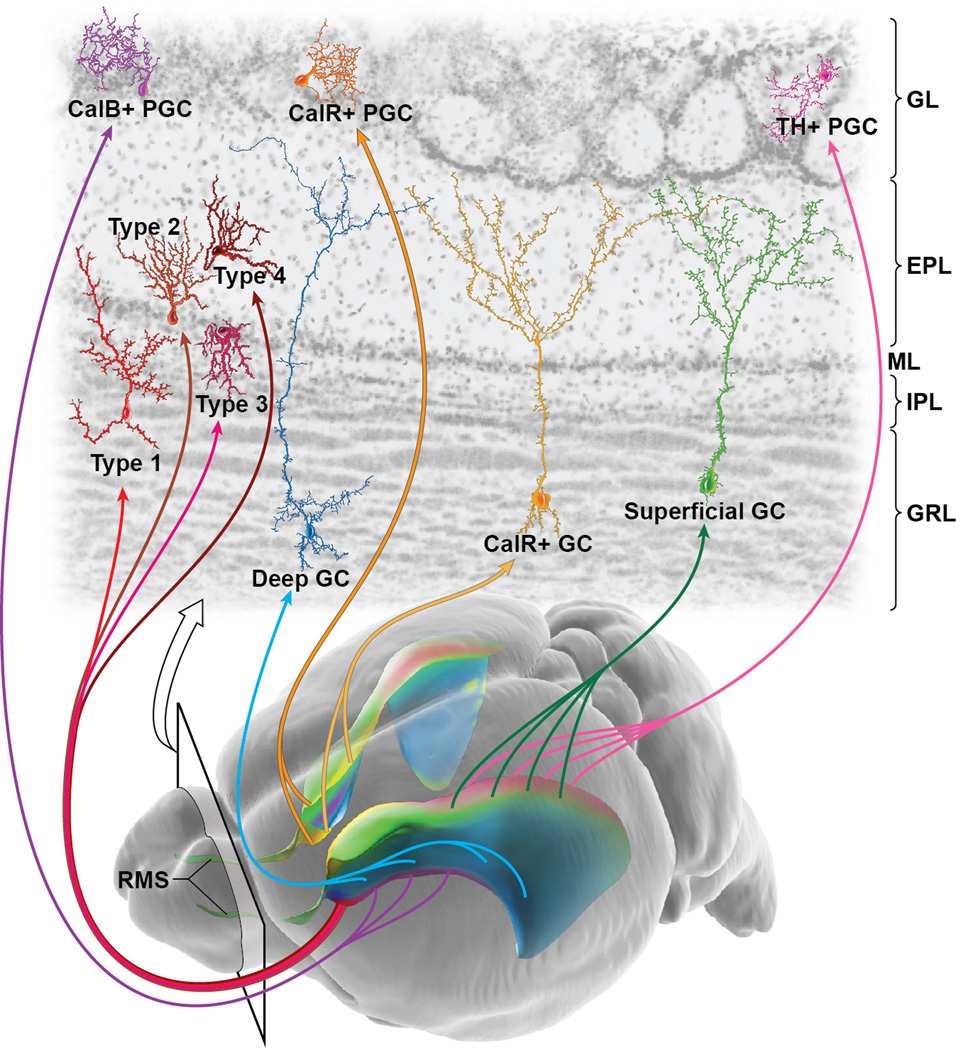
NSCs located in geographically distinct regions of the ventricle walls produce different types of OB neurons [21, 22] (Fig. 2). For instance, dorsal NSCs produce superficial granule cells (GCs) and anteriorly TH-positive periglomerular cells (PGCs), but few, if any, calbindin-positive PGCs. In contrast, ventral NSCs produce deep GCs and calbindin-positive but not tyrosine hydroxylase (TH)-positive periglomerular cells. Results from heterotopic grafting of NSCs (e.g., ventral NSCs transplanted into the dorsal V-SVZ) indicate that the regional differences of these NSCs are in large part cell-intrinsic (i.e., ventrally-derived NSCs grafted to the dorsal V-SVZ still produce calbindin-positive but not TH-positive PGCs). Thus, type B1 cells are not a homogeneous population of adult NSCs, and it is likely that these precursor cells will have significant epigenetic differences based on their physical location.
Figure 2. Regional organization of V-SVZ NSCs.
Oblique view of the adult mouse brain (bottom) with colorized lateral ventricles to indicate the regional organization of this major neurogenic niche. Cells born in different subregions of the adult V-SVZ migrate along the RMS into the olfactory bulb to give rise to unique types of interneurons [21]. Examples of 3 types of granular cells (green, superficial; yellow, calretinin superficial; blue, deep), 3 subtypes of periglomerular cells (pink, tyrosine hydroxylase; orange, calretinin; purple, calbindin) and 4 novel subtypes of interneurons derived from the most anterior V-SVZ [27] are indicated in the top section of the olfactory bulb. CalB, calbindin; CalR, calretinin; TH, tyrosine hydroxylase; PGC, periglomerular cell; GC, granule cell; GL, glomerular layer; EPL, external plexiform layer; ML, molecular layer; IPL, internal plexiform layer; GRL, granular layer.
OB interneurons are not only derived from the lateral wall of the lateral ventricle, but some specific subtypes are derived from the dorsal wall facing the cortex (pallium) [23, 24], the medial wall facing the septum and from within the RMS [21, 25, 26]. Thus, OB interneurons are derived from a very expansive postnatal germinal layer that relates to most major subdivisions of the embryonic telencephalon.
It remains unclear how many V-SVZ subregions exist and how many different types of OB interneurons are produced in adulthood. In addition to the six different subtypes of postnatally-born OB neurons that have been previously described [4], recent evidence has identified the genesis of four additional subtypes of OB interneurons from very restricted subregions of the anterior V-SVZ [27]. These new interneurons continue to be produced in postnatal and adult brain, migrate into the OB, and differentiate near the mitral cell layer. Although these new interneuron subtypes are produced in relatively low numbers as compared to GCs, their morphology and location suggests that they contribute uniquely to OB circuits. Thus, the adult V-SVZ is divided into subregions that are specialized for the production of distinct types OB interneurons. It remains to be determined whether the regional differences among the B1 cells directly relates to the positional identity of their embryonic radial glial cell origin. It is also unclear how these differences are maintained faithfully for long periods of time. For instance, is regional identity maintained by concomitant expansion of morphogen activity? Are cell intrinsic epigenetic mechanisms also required?
Activating the stem cell niche with blood vessels, ependyma, and neurons
Stem cells reside in a specialized microenvironment – or “niche” – that contributes to the regulation of proliferation and progenitor cell differentiation. Similar to other stem cell populations [28–30], NSCs of the V-SVZ are regulated by a multitude of niche signaling pathways including SHH, Wingless (Wnt), Notch, bone morphogenetic proteins (BMPs), and Ephrins (see [4, 31] for more extensive review). The local vascular plexus also appears to be a source of important extrinsic signals for the regulation of V-SVZ progenitor cells. Injection of tracer molecules into the peripheral blood stream reveals “leakiness” of the vasculature where clusters of proliferating C cells are found [32]. This finding is particularly interesting, given that the specific factors in the blood of young animals potentiates neurogenesis when transfused into older animals [33]. Some niche factors are derived from the endothelial cells themselves [34]. In particular, Betacellulin (BTC) is an EGF-like growth factor secreted by endothelial cells that is required for V-SVZ cell proliferation and neuroblast production [35]. Other important niche factors, such as stromal derived factor-1 (SDF1) [36] and pigment epithelium-derived factor (PEDF) [37, 38], are produced by both endothelia and ependymal cells.
By their close apposition to B1 cells, ependymal cells are likely important to the V-SVZ. Disruption of the ependymal cell layer by deletion of ANKYRIN3, a cytoskeletal adaptor protein found at the apical-lateral junctions of ependymal cells, results in in decreased neuroblast generation in the V-SVZ [39]. High levels of BMP signaling promotes glial differentiation of V-SVZ NSCs at the expense of neurogenesis, and V-SVZ ependymal cells secrete the low-density lipoprotein receptor-related protein 2 (LRP2), which acts as an endocytic receptor for BMP4 [40]. Mice null for LRP2 have increased BMP signaling in the V-SVZ, and neurogenesis is impaired. Given that ependymal cells also express Noggin [41], a secreted inhibitor of BMP proteins, it appears that BMP signaling is modulated for the control of neurogenesis from the adult V-SVZ niche.
A number of different neurotransmitters play a role in the regulation of V-SVZ neurogenesis. For instance, serotonin (5-HT) acts through receptors in the V-SVZ, regulating cell proliferation and OB neurogenesis [42, 43]. Recent data suggests that most, if not all, 5-HT axons are supraependymal and form a dense plexus contacting both ependymal and type B1 cells [44] (Fig. 1). In the postnatal V-SVZ, young neuroblasts spontaneously release gamma-aminobutyric acid (GABA), activating GABAA-receptors on precursor cells. This GABA-dependent depolarization of neural precursors inhibits cell proliferation and neuronal differentiation [45, 46]. Thus, GABA may function in part as a negative-feedback signal derived from neuroblasts, down-regulating their own production. Interestingly, type B1 and C cells secrete the diazepam binding inhibitor protein (DBI), which competitively inhibits GABA from binding to GABA receptors, increasing the proliferation and neurogenesis of the V-SVZ [47].
The V-SVZ also receives dopaminergic innervation from the midbrain, with type C cells being the predominant cell type expressing D2 receptors, but D3 receptors may also be involved in V-SVZ neurogenesis [48]. Dopaminergic denervation results in decreased SVZ proliferation and OB neurogenesis, while administration of the dopamine precursor levodopa restore SVZ proliferation to near normal levels [49]. The dopamine-induced activation of V-SVZ neurogenesis is in part mediated through EGF-dependent mechanisms [50].
There is also a population of choline acetyltransferase (ChAT)(+) neurons in the V-SVZ niche (Fig. 1), and these neurons are morphologically distinct from other striatal neurons [51]. Optogenetic inhibition and stimulation of subependymal ChAT(+) neurons increases neurogenic proliferation in part by synergizing with fibroblast growth receptor activation. Taken together, these studies indicate that the V-SVZ is recipient of neural activity and therefore integrated into the electrophysiological network activity of the adult brain. However, the logic behind the activation of different neuronal pathways and the regulation of neuronal production in the V-SVZ remains unknown.
Cell intrinsic signals for long-term and complex patterns of neurogenesis
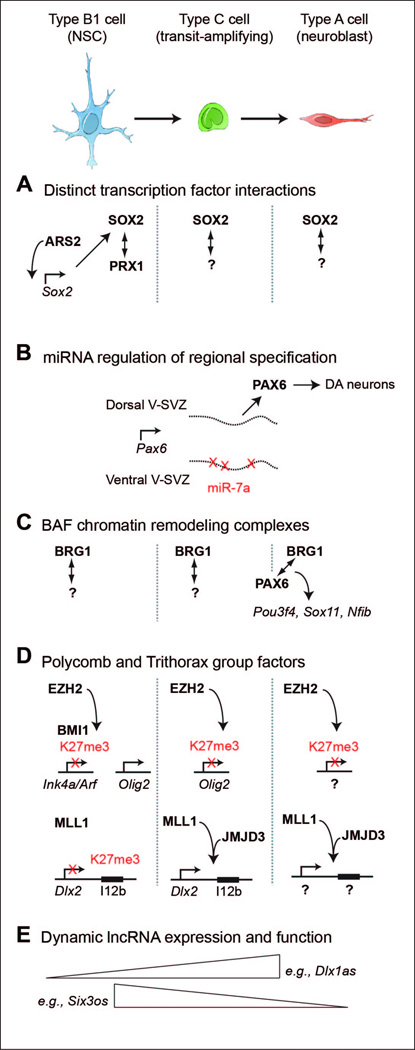
SOX2 is a sequence-specific DNA binding transcription factor with many critical functions in a wide variety of cell populations throughout development [52]. How SOX2 performs such diverse roles in different cell types is not well understood, and recent studies of the V-SVZ lineage have shed light on this important and complex issue (Fig. 3A). In the V-SVZ, SOX2 is expressed in essentially all V-SVZ cell types, including B1 cells [53]. B1 cells express the PRX1 homeobox transcription factor, and PRX1 physically interacts with SOX2 [54]. Prx1 deficient V-SVZ NSCs have defective self-renewal in vitro, suggesting that PRX1 is a SOX2 co-activator that specifies NSC self-renewal. SOX2 also physically interacts with the TLX transcription factor, regulating the expression of Tlx, which is required for B1 cell proliferation [55, 56]. Both SOX2 and TLX can bind the Tlx2 promoter, and while TLX normally represses its own expression, SOX2 positively regulates Tlx transcription, suggesting that SOX2 maintains Tlx expression via antagonism of a Tlx negative feedback loop.
Figure 3. Insights into cell intrinsic regulators of V-SVZ neurogenesis.
At top, a schematic of the V-SVZ neurogenic lineage. B1 cells (blue) give rise to transit-amplifying C cells (green) that give rise to A cells (red) that migrate to the OB where they differentiate into different types of interneurons. In panels below, vertical dotted lines (when present) separate the expression and action of the factors into these cell types of the neurogenic lineage. (A) While SOX2 is expressed in throughout the V-SVZ neurogenic lineage and likely performs distinct functions in each cell type, ARS2 [57] and PRX1 [54] are B1 cell-specific and required for NSC self-renewal. Potential co-factors for SOX2 in the C and A cells are not yet known. (B) Pax6 mRNA is transcribed in cells along the dorsal to ventral extent of the V-SVZ, but expression of miR-7a in the ventral regions represses Pax6 translation [67]. (C) BRG1 and PAX6 interact and are required for neurogenic gene expression [73]. (D) Polycomb factors EZH2 and BMI1 are required to repress Ink4a/Arf to enable NSC proliferation, but during differentiation, EZH2 activity becomes localized to Olig2 and this transcriptional repression is required for neurogenesis [87]. How EZH2 is “targeted” to specific loci during differentiation remains poorly understood. Trithorax factor MLL1 is required to antagonize the transcriptional repression of Dlx2 [88], possibly in cooperation with the H3K27me3-demethylase JMJD3 acting at the I12b intergenic enhancer (black rectangle) of Dlx2 [90]. Other promoters and enhancers regulated by MLL1 and JMJD3 in V-SVZ cells remain to be described. (E) Many lncRNAs are regulated during neurogenesis and some are required for NSC function [94].
In V-SVZ NSCs, Sox2 expression requires Arsenite-resistance protein 2 (Ars2) [57]. Ars2-deletion results in the loss of NSC self-renewal, and this defect can be rescued by Sox2 overexpression. Interestingly, while orthologs of Ars2 function in miRNA biogenesis, ARS2 in V-SVZ NSCs acts as a transcription factor, binding to an enhancer element to activate Sox2 expression.
Combinations of transcription factors also regulate neuronal-glial lineage specification. C cells and some B1 cells express the basic helix-loop-helix (bHLH) transcription factor ASCL1 [58], and Ascl1 is required for both neuronal and oligodendroglial lineages [59]. Some C cells co-express bHLH factor Neurogenin 2 (Ngn2), and overexpression of Ngn2 downregulates Ascl1, inducing the production of calretinin-postive OB neurons [60]. Another bHLH factor, Olig2, is expressed in some type B1 cells and type C cells [61], and overexpression of Olig2 appears to repress the neuronal lineage and promote oligodendrogliogenesis [62]. Conversely, expression of a dominant-negative form of OLIG2 inhibits oligodendrocyte production and induces the ectopic expression of neuronal markers [63].
While some transcription factors such as Neurod1 are required for the genesis of OB neurons in general [64] [65], emerging evidence indicate that region-specific expression of transcription factors underlies the generation of the diverse populations of OB interneurons. For instance, the homeobox gene Emx1 is expressed primarily in the developing pallium. However, cells derived from progenitors expressing Emx1 also generate calretinin-positive superficial GCs and PGC interneurons [23]. Along these lines, ventral V-SVZ cells expressing Nkx6.2 and septal precursors with Zic expression generate some of the distinct OB neuron subtypes discussed above [27] (Fig. 2).
The production of dopaminergic PGCs requires the homeobox gene Paired box 6 (Pax6), indicating that some transcription factors specify OB neuron subtypes [60]. Neuroblasts null for the zinc-finger transcription factor Sp8 have increased Pax6 expression and defective production of non-dopaminergic PGCs and GCs [66]. This suggests that Sp8 normally represses Pax6, shifting the neuroblast fate from dopaminergic PGC to non-dopaminergic interneurons. While Pax6 mRNA is present in V-SVZ cells along the dorsal-ventral extent, PAX6 protein expression is restricted to the dorsal regions. This post-translational regulation of PAX6 expression was determined by regional expression of miR-7a (Fig. 3B), suggesting that microRNAs play a key role in determining the regional heterogeneity of the V-SVZ [67]. Furthermore, by regulating other transcripts such as Tlx and Sox9, microRNAs are important for the maintenance of NSC self-renewal and neuronal lineage specification [68, 69]. Future combinatorial loss-of-function studies may be able to decipher a potential transcriptional “code” that determines the multitude of OB interneuron subtypes.
Epigenetic mechanisms for the maintenance of neurogenic competence
It has been suggested that B1 cells, like other stem cell populations, can self-renew and have the ability to generate multiple cell types. Conceptually, this cellular state of “stemness” involves the expression of certain genes, repression of other specific loci, and transcriptional plasticity of genes necessary for lineage specification. Such complex patterns of transcriptional regulation involve the structure and function of chromatin. The basic subunit of chromatin is the nucleosome, which consists of 146 base pairs of DNA wrapped approximately twice around an octamer of the four core histone proteins (H3, H4, H2A, H2B). Chromatin states are regulated by both non-covalent and covalent changes that can either promote or repress transcription [70], and chromatin-based regulation is critical to V-SVZ neurogenesis [71].
Brama (Brm) associated factor (BAF) chromatin-remodeling complexes utilize ATP hydrolysis to alter chromatin structure, generally facilitating transcription. Mammalian BAF complexes consist of an ATPase subunit encoded by Brg1 or Brm and up to twelve other BAF subunits [72]. In cultured NSCs, PAX6 interacts with BRG1, and conditional deletion of Brg1 in V-SVZ NSCs results in defective neurogenesis [73]. Gene expression and chromatin analysis suggest that PAX6 interacts with BRG1-containing BAF complexes for the activation of a neurogenic transcriptional program (Fig. 3C).
Histone acetylation is a covalent chromatin modification associated with active transcription [74], and multiple histone deacetylases (HDACs) play key roles in V-SVZ neurogenesis [75–78]. In cultured V-SVZ cells, TLX recruits HDAC5 to the promoters of the Cdkn1a and Pten cell cycle regulators, and disruption of the interaction between TLX and HDAC5 with a TLX peptide results in increased Cdkn1a and Pten expression and reduced NSC proliferation. Thus, it appears that the interaction between HDACs and specific transcription factors is required for neurogenesis.
While DNA methylation is well known for its role in transcriptional repression at gene promoters, DNA methylation also occurs in non-promoter genomic regions. DNMT3A is a de novo methyltransferases [79] that is prominently expressed in the V-SVZ and is required for non-promoter DNA methylation, which facilitates the transcription of key neurogenic genes including Dlx2 [80].
The Polycomb repressive complex 2 (PRC2) contains EZH2, which catalyzes histone 3 lysine 27 trimethylation (H3K27me3) [81]. BMI1 and RING1B are components of Polycomb repressive complex 1 (PRC1) that recognizes H3K27me3 and mediates transcriptional silencing. In the adult V-SVZ, Bmi1-mediated gene silencing is required for self-renewal of cultured NSCs in part via repression of Ink4a/Arf [82–86]. While Bmi1 is widely expressed in mature neurons and astrocytes, during postnatal development, EZH2 becomes restricted to the adult NSC populations and their young daughter cells [87]. Thus, in both the mouse and human brain, the expression of EZH2 is a distinguishing feature of neurogenic astroglia. In mouse V-SVZ, EZH2 is required for neurogenesis independent of its role in precursor cell proliferation, as Ink4a/Arf-deficiency in Ezh2-deleted V-SVZ cells rescues cell proliferation, but neurogenesis remains defective. Olig2 is a direct target of EZH2, and repression of this transcription factor is critical for neuronal differentiation (Fig. 3D). Thus, EZH2 is a PcG factor that distinguishes B1 cells from non-neurogenic astrocytes and regulates both self-renewal and neuronal differentiation. Of note, self-renewal is a cellular property that has been primarily studied in vitro, and it remains to be determined whether such cell culture assays directly reflect the behavior of B1 cells in vivo.
In contrast to the transcriptional repression mediated by PcG factors, trxG complexes are positive regulators of gene expression. Cells of the V-SVZ neurogenic lineage express the trxG member Mixed lineage leukemia-1 (Mll1), and Mll1-deletion in B1 cells inhibits neurogenesis – but not glial cell differentiation [88]. Mll1 is required for neurogenic gene expression, and in particular, Dlx2 is a direct target. In Mll1-deleted cells, the Dlx2 locus is enriched for H3K27me3, correlating with the lack of Dlx2 upregulation. These data suggested that MLL1 is required for the activity of an H3K27-specific demethylases such as UTX or JMJD3 at specific neurogenic transcriptional regulatory elements.
Like gene promoters, the chromatin state of transcriptional enhancers can be ‘poised’ by the presence H3K27me3 [89], and in SVZ NSCs, the I12b Dlx2 enhancer has a poised chromatin state [90]. Without JMJD3, the I12b enhancer remains enriched with H3K27me3, and Dlx2-dependent neurogenesis fails. Thus, in addition to acting at promoter regions, JMJD3 appears to activate enhancers during SVZ lineage specification. Interestingly, in Mll1-deleted cells, JMJD3 is not enriched at I12b, indicating that MLL1 is required – either directly or indirectly – for the localization of JMJD3 at a key regulatory enhancer. Furthermore, analysis of ChIP-seq data indicates that in the developing cortex, JMJD3 is enriched at many thousands of neural enhancers [90], suggesting that this H3K27me3-specific demethylase acts at enhancers during brain development, as well.
Emerging evidence indicate that long non-coding RNAs (lncRNAs) – transcripts >200 nucleotides with no evidence of protein coding potential – are involved in the targeting of EZH2, MLL1, and other chromatin-modifying complexes [91–93]. Recently, lncRNAs have been associated with specific V-SVZ cell types and lineage specification [94]. Integration of multiple genome-wide approaches including RNA-seq, RNA CaptureSeq, ChIP-seq, and custom microarrays identified ~100 lncRNAs with potential roles in V-SVZ neurogenesis. While knockdown of lncRNA Six3os reduced both neurogenesis and the production of OLIG2-lineage cells, the knockdown of Dlx1as selectively reduced neuronal differentiation from V-SVZ NSC cultures, suggesting that lncRNAs play a role in neuronal-glial lineage specification (Fig. 3E). Ongoing V-SVZ studies may provide novel insights into the in vivo function and molecular mechanism of lncRNAs, which is a prominent knowledge gap in this emerging field.
The human brain V-SVZ
Recent studies have revealed intriguing differences between the rodent and human V-SVZ. In young children, the V-SVZ contains many DCX-positive cells, and there is a prominent RMS to the OB [95]. Furthermore, the brain of infants also has a medial migratory stream of young neurons from the V-SVZ to the medial prefrontal cortex, which is a migratory path not evident in the rodent brain. After 18 months of age, few DCX-positive cells are observed in the human V-SVZ. Instead, there is a prominent gap layer (GAP) consisting of a dense network of interconnected processes from astrocytes and ependymal cells [96, 97]. Adjacent to the GAP is a cellular “ribbon” containing astrocytes, some of which have been inferred to have NSC properties in vitro. While very few young neurons are observed in the GAP, recent evidence suggests that precursor cells migrate into the adult striatum and differentiate into local interneurons [98]. However, the claim of neurogenesis in adult human striatum is still controversial, as a very recent study suggests that all interneurons in the adult human and monkey striatum arise during development and not in the adult [99]. Thus, while the V-SVZ clearly has a prominent role during early childhood, the role of the adult human V-SVZ remains elusive, and available evidence indicates that this neurogenic niche may be largely dormant in the adult human brain. Future studies are needed to better understand the differences between the rodent and human V-SVZ.
Conclusion
Over the past two decades, in vivo and in vitro studies of the V-SVZ have revealed the experimental advantages of studying a germinal niche that remains active throughout adult life. As discussed above, neurogenesis in the V-SVZ involves an intriguing interplay between niche signals including epithelial and vascular-derived signals, as well as activity-dependent neurotransmitters. Furthermore, the maintenance of long-term neurogenesis and regional NSC specification has been discovered to involve complex interactions between transcription factors, chromatin remodeling/modifying enzymes, and different classes of non-coding RNAs. The V-SVZ should continue to serve as an exciting model system for the discovery of novel molecular and cellular interactions that regulate neurogenesis and gliogenesis (see Box 1). Given that adult NSCs possess many characteristics of their embryonic radial glial precursors, continued work in the V-SVZ is likely to reveal mechanistic themes relevant to embryonic and postnatal brain development. The V-SVZ may thus contribute importantly to our understanding of human disease and could potentially suggest therapeutic strategies.
Box 1. OUTSTANDING QUESTIONS.
NSCs in the adult V-SVZ retain regional identity related to their embryonic origin, and this specification appears to be largely cell-intrinsic. How do NSCs maintain such complex developmental information for long periods of time? During early embryonic development, such patterns are established by extrinsic factors such as SHH, but it seems likely that cell-intrinsic, epigenetic mechanisms also play key roles in this important developmental issue. Understanding how V-SVZ NSC retain regional identity may provide important insights into how the human embryonic brain - which grows much larger -maintains complex patterns of gene expression over the several months of its development.
V-SVZ NSCs are frequently described as being multipotent - capable of generating neurons, astrocytes, and oligodendrocytes. However, a recent in vitro imaging study indicates that NSCs acutely isolated from the V-SVZ exclusively generate either neurons or oligodendrocytes [100]. Do V-SVZ NSCs in vivo behave in a similarly lineage-restricted manner?
Intriguingly, V-SVZ neurogenesis is regulated by factors from the vasculature and neuronal axons. The “logic” of such regulation remains to be discovered. Factors in the peripheral blood appear to be critical to V-SVZ neurogenesis, which would suggest that non-CNS organs provide key input into neural development - possibly even during development. The continued identification of such factors will provide exciting new clues about how the body as a whole can contribute to the development and function of the brain. A deeper understanding of the neurons and circuits that innervate the V-SVZ may reveal how specific brain activity can influence the cellular composition of the stem cell niche as well as the OB target.
While multiple studies of chromatin regulators have demonstrated their critical importance to NSCs and their differentiation into specific neural cell types, details about how they orchestrate such complex biological processes remains to be discovered. For instance, the H3K27me3 methyltransferase EZH2 is expressed in the entire SVZ neurogenic lineage, and it appears that this Polycomb group repressive factor is targeted to specific loci such as Olig2 during differentiation. What are the molecular mechanisms that underlie such dynamic targeting of EZH2? Similarly, how are H3K27-specific demethylases such as JMJD3 targeted to neural gene transcriptional regulatory elements?
At this point, it is clear that lncRNAs can have diverse biological functions, but there are currently few lncRNAs with well-understood developmental roles and molecular mechanisms. The relatively stable architecture of the V-SVZ, and the ability to isolate and culture V-SVZ NSCs for molecular studies, presents a tractable model system in which to study such fundamentally important questions at the cellular and molecular level.
Highlights.
Neural stem cells retain regional identity into adulthood.
Spinal fluid, vasculature, and neuronal activity control adult V-SVZ neurogenesis.
New roles for chromatin modifiers and non-coding RNAs are discovered in the V-SVZ.
ACKNOWLEDGEMENTS
This work was supported by NIH grant DP2-OD006505, VA grant 1I01 BX000252, a Sontag Foundation Award, and a generous gift from the Shurl and Kay Curci Foundation to D.A.L. A.A.B is the Heather and Melanie Muss Endowed Chair and is supported by NIH grants HD032116, NS28478, and a generous gift from the JG Bowes Foundation. We thank Joseph Elsbernd for his helpful edits and comments on the manuscript and Ken Probst for illustrations.
GLOSSARY
- Regional identity
the developmental specialization of precursor cells based on their physical location
- Epigenetics
the study of heritable patterns of gene expression that do not involve changes to the DNA sequence
- Chromatin
the dynamic polymer of DNA and histone proteins. The nucleosome – approximately 146 bp of DNA wrapped approximately twice around an octamer of the four core histone proteins (H2A, H2B, H3, H4) – is the basic subunit of chromatin. Chromatin can undergo non-covalent and covalent changes, which corresponds to differences in local transcriptional activity. Some of these chromatin-based changes are heritable through cell division
- Non-coding RNA
While only 1–2% of the mammalian genome codes for protein, approximately 80% of the genome is transcribed, and most of these transcripts have essentially no protein coding potential. While some non-coding RNAs such as microRNAs have been studied in detail, long non-coding RNAs (lncRNAs) – those longer than 200 nt – are less understood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- 2.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuentealba LC, et al. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto M, et al. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J Neurosci. 2014;34:5788–5799. doi: 10.1523/JNEUROSCI.0674-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepousez G, et al. The impact of adult neurogenesis on olfactory bulb circuits and computations. Annual review of physiology. 2013;75:339–363. doi: 10.1146/annurev-physiol-030212-183731. [DOI] [PubMed] [Google Scholar]
- 7.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 8.Heins N, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 9.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Codega P, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mich JK, et al. Prospective identification of functionally distinct stem cells and neurosphere-initiating cells in adult mouse forebrain. eLife. 2014;3:e02669. doi: 10.7554/eLife.02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponti G, et al. Lineage progression from stem cells to new neurons in the adult brain ventricular-subventricular zone. Cell Cycle. 2013:12. doi: 10.4161/cc.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doetsch F, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 16.Conover JC, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 17.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Current topics in developmental biology. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihrie RA, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, et al. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Buylla A, et al. The Heterogeneity of Adult Neural Stem Cells and the Emerging Complexity of Their Niche. Cold Spring Harbor symposia on quantitative biology. 2008 doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- 23.Kohwi M, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso M, et al. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 2008;28:11089–11102. doi: 10.1523/JNEUROSCI.3713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergano-Vera E, et al. Generation of GABAergic and dopaminergic interneurons from endogenous embryonic olfactory bulb precursor cells. Development. 2006;133:4367–4379. doi: 10.1242/dev.02601. [DOI] [PubMed] [Google Scholar]
- 27.Merkle FT, et al. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17:207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978:7–25. [PubMed] [Google Scholar]
- 29.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs E, et al. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 31.Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Gaviro MV, et al. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci U S A. 2012;109:1317–1322. doi: 10.1073/pnas.1016199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez-Castillejo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 38.Andreu-Agullo C, et al. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 39.Paez-Gonzalez P, et al. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajera CR, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- 41.Lim DA, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 42.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 43.Banasr M, et al. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 44.Tong CK, et al. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14:500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernando RN, et al. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfonso J, et al. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell. 2012;10:76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, et al. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114:750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 50.O’Keeffe GC, et al. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paez-Gonzalez P, et al. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci. 2014;17:934–942. doi: 10.1038/nn.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis P, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Developmental neuroscience. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 54.Shimozaki K, et al. Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci. 2013;33:4066–4075. doi: 10.1523/JNEUROSCI.4586-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu HK, et al. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimozaki K, et al. SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem. 2012;287:5969–5978. doi: 10.1074/jbc.M111.290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andreu-Agullo C, et al. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature. 2012;481:195–198. doi: 10.1038/nature10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohwi M, et al. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roybon L, et al. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci. 2009;29:232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 61.Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hack MA, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 63.Marshall CA, et al. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–7298. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Z, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutin C, et al. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A. 2010;107:1201–1206. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waclaw RR, et al. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 67.de Chevigny A, et al. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat Neurosci. 2012;15:1120–1126. doi: 10.1038/nn.3142. [DOI] [PubMed] [Google Scholar]
- 68.Cheng LC, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi Y, et al. MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci. 2010;30:14931–14936. doi: 10.1523/JNEUROSCI.4280-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Gonzales-Roybal G, Lim DA. Chromatin-based epigenetics of adult subventricular zone neural stem cells. Frontiers in genetics. 2013;4:194. doi: 10.3389/fgene.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ninkovic J, et al. The BAF complex interacts with Pax6 in adult neural progenitors to establish a neurogenic cross-regulatory transcriptional network. Cell Stem Cell. 2013;13:403–418. doi: 10.1016/j.stem.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davie JR, Hendzel MJ. Multiple functions of dynamic histone acetylation. J Cell Biochem. 1994;55:98–105. doi: 10.1002/jcb.240550112. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- 76.Montgomery RL, et al. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foti SB, et al. HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int J Dev Neurosci. 2013 doi: 10.1016/j.ijdevneu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Jawerka M, et al. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron glia biology. 2010;6:93–107. doi: 10.1017/S1740925X10000049. [DOI] [PubMed] [Google Scholar]
- 79.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature reviews. Genetics. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu H, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molofsky AV, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fasano CA, et al. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes Dev. 2009;23:561–574. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He S, et al. Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo. Dev Biol. 2009;328:257–272. doi: 10.1016/j.ydbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang WW, et al. Distinct and separable roles for EZH2 in neurogenic astroglia. eLife. 2014;3:e02439. doi: 10.7554/eLife.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim DA, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rada-Iglesias A, et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park DH, et al. Activation of Neuronal Gene Expression by the JMJD3 Demethylase is Required for Postnatal and Adult Brain Neurogenesis. Cell reports. 2014:8. doi: 10.1016/j.celrep.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bertani S, et al. The noncoding RNA Mistral activates Hoxa6 and Hoxa7 expression and stem cell differentiation by recruiting MLL1 to chromatin. Mol Cell. 2011;43:1040–1046. doi: 10.1016/j.molcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramos AD, et al. Integration of Genome-wide Approaches Identifies lncRNAs of Adult Neural Stem Cells and Their Progeny In Vivo. Cell Stem Cell. 2013;12:616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 97.Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 98.Ernst A, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 99.Wang C, et al. Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J Neurosci. 2014;34:10906–10923. doi: 10.1523/JNEUROSCI.1758-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ortega F, et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nature cell biology. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]