Abstract
Plants developed under constant high (> 85%) relative air humidity (RH) have larger stomata that are unable to close completely. One of the hypotheses for the less responsive stomata is that the plants have reduced sensitivity to abscisic acid (ABA). Both ABA and darkness are signals for stomatal closure and induce the production of the secondary messenger hydrogen peroxide (H2O2). In this study, the ability of Vicia faba plants developed in moderate or high RH to close the stomata in response to darkness, ABA and H2O2 was investigated. Moreover, the ability of the plants to produce H2O2 when treated with ABA or transferred to darkness was also assessed. Our results show that the ABA concentration in moderate RH is not increased during darkness even though the stomata are closing. This indicates that stomatal closure in V. faba during darkness is independent of ABA production. ABA induced both H2O2 production and stomatal closure in stomata formed at moderate RH. H2O2 production, as a result of treatment with ABA, was also observed in stomata formed at high RH, though the closing response was considerably smaller as compared with moderate RH. In either RH, leaf ABA concentration was not affected by darkness. Similarly to ABA treatment, darkness elicited both H2O2 production and stomatal closure following plant cultivation at moderate RH. Contrary to this, neither H2O2 production nor stomatal closure took place when stomata were formed at high RH. These results suggest that the reduced stomatal response in plants developed in continuous high RH is caused by one or more factors downstream of H2O2 in the signaling pathway toward stomatal closure.
Keyword: Abscisic acid, hydrogen peroxide, darkness, relative air humidity, stomatal closure, guard cells
Introduction
The stomatal complex consists of two guard cells surrounding the stomatal pore and functions as a gate for CO2 uptake for photosynthesis and transpirational water loss. Through regulation of the stomatal opening the need for CO2 uptake and water preservation can be closely balanced.1
Previous studies have shown that plants developed under constant high (> 85%) relative air humidity (RH) have larger stomata that are unable to close completely in response to closing stimuli like desiccation, darkness and the hormone abscisic acid (ABA).2-4 These plants therefore have reduced ability to preserve water, leading to higher water loss.5 Similar results have been found in micropropagated plants where the RH is high and leaf cuttings rooted at high RH.6,7
The ABA levels in the leaves are dependent on the ABA transported from the roots and the ABA that is produced in the leaves themselves.8,9 Also, the degradation of ABA to phaseic acid (PA) and conjugation with glucose to form ABA-glucose ester (ABA-GE) determines the ABA concentration in the leaves.10,11 The stomatal malfunctioning found in plants developed under high RH has previously been hypothesized to be a result of low ABA levels.12 It has recently been shown that plants developed in high RH are able to produce large amounts of ABA during desiccation.4,13 It is therefore likely that the reduced ability to close the stomata is a result of an impaired ABA signaling pathway.14
ABA in the guard cells is percepted by PYRABACTIN RESISTANCE (PYR)/PYR1-LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR).15,16 This in turn induces the formation of reactive oxygen species (ROS). Hydrogen peroxide (H2O2) is formed first, most likely by NADPH oxidase, which generates superoxide and H2O2.17 H2O2 induces nitrogen oxide (NO) production.18 Both H2O2 and NO are essential for stomatal closure and removal of either result in reduced stomatal closure.18-20 Downstream of NO in the signaling pathway is the influx of Ca2+ into the cytosol, which activates anionout channels and K+out channels and inhibits the proton pumps.21 This depolarizes the plasma membrane and decreases the turgor pressure in the guard cells leading to stomatal closure. It remains unknown whether the decreased stomatal responsiveness to ABA found in high RH grown plants is due to early signaling events (i.e., perception), the production of secondary messengers (e.g., H2O2) or stomatal closure mechanism.
During darkness most plants close the stomata in order to retain water and rehydrate.1 Previous studies have also shown that the extent of stomatal closure during darkness is dependent on the environmental conditions, such as temperature, drought and air humidity.22,23 Both Arabidopsis thaliana and Rosa x hybrida growing in high RH (> 85%) have reduced dark-induced stomatal closure compared with plants growing in moderate RH.4,22 The signaling pathway for stomatal closure in darkness does not necessarily involve ABA, but H2O2 is still required.17 It is possible that H2O2 production during darkness is affected by growth RH and is decreased in high RH.
The aim of this study was to investigate if plants developed under constant high RH have reduced sensitivity to ABA. To shed light on this, the ability of the plants to initiate H2O2 production and close the stomata in response to ABA or darkness was tested. The importance of increased ABA concentrations to initiate stomatal closure during darkness was also assessed. Previous work on responses of the stomatal complex to RH and possible signaling molecules involved has only been performed on intact plants or detached leaves.13 In this study we used epidermal peels from Vicia faba, which emit very little auto-fluorescence that could interfere with the fluorescence analysis, to study the stomatal responses of plants grown at high and moderate RH to darkness, ABA and H2O2.
Results
Development of Vicia faba leaves in high RH resulted in larger stomata with reduced responses to closing stimuli
The effect of RH on stomata was investigated in V. faba in order to study the signaling involved. When developed under high RH V. faba stomata in epidermal peels were larger in size compared with stomata developed under moderate RH (P < 0.001, Fig. 1). The average length of the stomatal pore in moderate and high RH was 57 µm and 48 µm, respectively (Fig. 2).
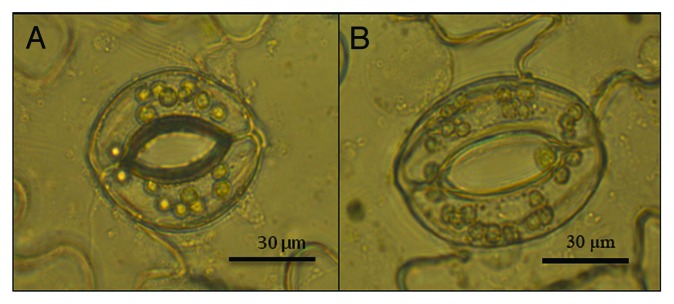
Figure 1. Light microscope images of stomata in moderate (60%; A) and high (90%; B) relative air humidity (RH). The images are of epidermal peels from the abaxial side of V. faba leaves in MES buffer.
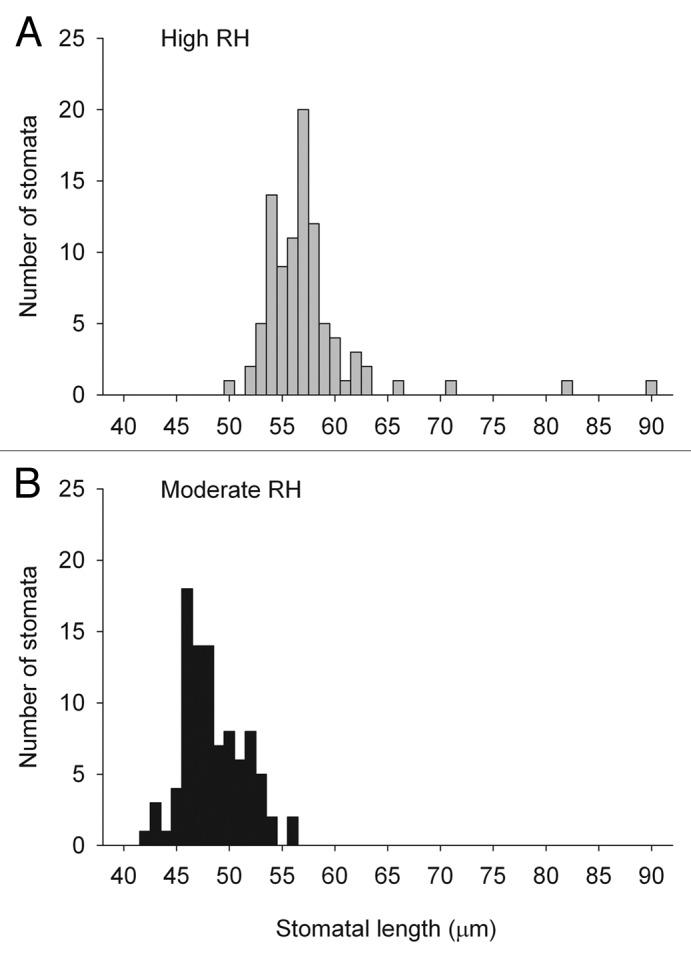
Figure 2. Frequency distribution of stomatal pore length. Stomatal pore length was measured on epidermal peels of V. faba developed in high (90%; A) and moderate (60%; B) relative air humidity (RH). n = 93.
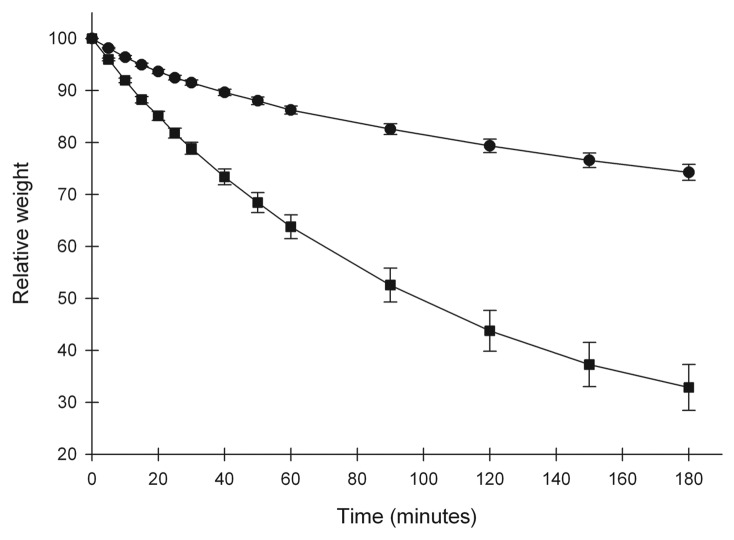
A desiccation test was performed to test the ability of V. faba plants from moderate and high RH to close the stomata. A significant difference in water loss was found after only 5 min of desiccation (P < 0.001). The difference in water loss increased throughout the test and after 180 min the plants from high RH had lost approximately 70% of their initial weight (Fig. 3). In contrast, plants from moderate RH had only lost about 20% of their initial weight (Fig. 3).
Figure 3. Weight loss of detached V. faba leaves, developed under moderate (60%; circles) and high (90%; squares) relative air humidity (RH), during 180 min of desiccation in a dry environment (40% RH), n = 11. Mean values ± SE are shown.
Stomatal closure during darkness was measured using imprints of the abaxial side of the leaves. There was no significant difference in stomatal aperture between the two RH treatments during the light period. At this time point the stomatal aperture was in average 10.5 µm in moderate RH and 10.9 µm in high RH (Fig. 4A). During the dark period the aperture was significantly smaller in plants grown in moderate RH compared with plants grown in high RH (P < 0.001). Plants grown in moderate RH closed the stomata during darkness to an aperture of 7.5µm, while the plants grown in high RH kept the stomata wide open with an aperture of 11.4µm (Fig. 4A).
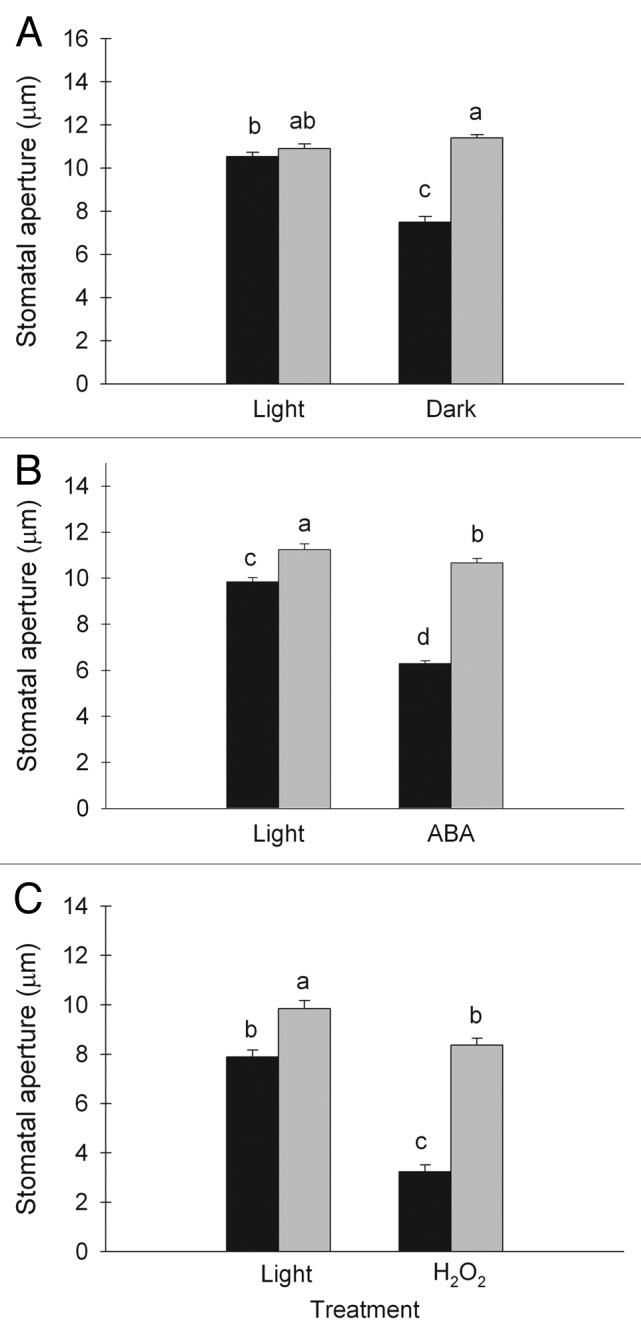
Figure 4. Stomatal apertures were measured on imprints or epidermal peels taken from leaves developed in moderate (60%; black bars) and high (90%; gray bars) relative air humidity (RH). Measurements during the dark period and a control during the light period were done using imprints (A). Epidermal peels were used to assess the effect of abscisic acid (ABA) (B) and H2O2 (C) on stomatal aperture. Different letters indicate significant differences, n = 160 (A), 216 (B), 68–75 (C). Mean values ± SE are shown.
To evaluate the involvement of ABA and H2O2 as signaling molecules in stomatal response to RH we measured the stomatal aperture in epidermal peels harvested from plants grown at moderate and high RH after treatment with ABA and H2O2. The average stomatal aperture in epidermal peels from plants grown under moderate RH was 9.8 µm and 7.9 µm in light before respectively ABA and H2O2 treatment (Fig. 4B and C). The stomata closed to 6.3 and 3.2 µm after respectively ABA and H2O2 treatment (Fig. 4B and C). The stomatal aperture in epidermal peels from plants grown under high RH prior to respectively ABA and H2O2 treatment was 11.2 µm and 9.9 µm in light and 10.7 µm and 8.4 µm after the ABA and H2O2 treatment (Fig. 4B and C). Therefore, both treatments showed stomatal closure in response to ABA and H2O2. However, plants from high RH only exhibited a 5% reduction in stomatal aperture in response to ABA (P < 0.050), while plants from moderate RH showed a 36% reduction (P < 0.001). Similarly, plants from high RH only had a 15% reduction in stomatal aperture in response to H2O2 (P < 0.001), while plants from moderate RH exhibited a 59% reduction (P < 0.001). This shows that plants developed in high RH responded to a smaller extent to ABA and H2O2 than plants developed in moderate RH.
Plants developed in high RH increased H2O2 production in response to ABA but not during darkness
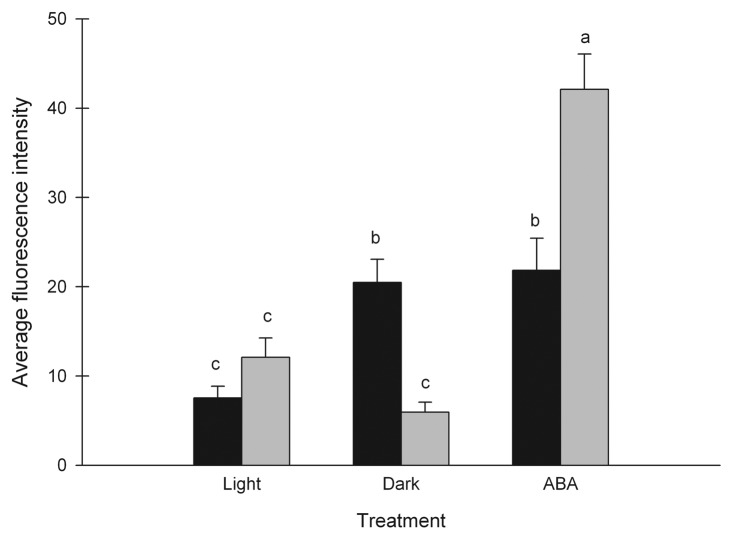
Induction of stomatal closure by ABA involves production of H2O2.18 To assess if the plants had a reduced ability to initiate the pathway toward stomatal closure H2O2 levels in the guard cells was quantified in plants grown in moderate or high RH kept in light, darkness and after ABA treatment.
The light treatment was used as a control to represent the amount of H2O2 in open stomata. Under light there was no significant difference in the amount of H2O2 between high and moderate RH (Fig. 5). After exposure to darkness, the amount of H2O2 was significantly higher in guard cells of moderate RH-grown plants compared with those from high RH-grown plants (P < 0.001). Also, compared with the light control the amount of H2O2 increased significantly during darkness in guard cells from moderate RH (P < 0.001), but did not change significantly in guard cells from high RH (Fig. 5). Furthermore, ABA treatment of guard cells from moderate as well as high RH-grown plants resulted in significantly higher H2O2 levels compared with the control (P < 0.001, Fig. 5). After the ABA treatment the amount of H2O2 was significantly higher in guard cells from high RH, than in guard cells from moderate RH (P < 0.001). In summary, the H2O2 levels in guard cells from moderate RH increased after treatment with ABA and in darkness whereas in high RH the H2O2 levels increased in response to ABA treatment, but not in darkness.
Figure 5. Levels of H2O2 in the guard cells of epidermal peels from plants grown in moderate (60%; black bars) or high (90%; gray bars) relative air humidity (RH) after incubation in light (control), darkness and after treatment with abscisic acid (ABA) as measured by fluorescence microscopy after addition of dichlorodihydrofluorescein diacetate. Different letters indicate significant differences, n = 72–98. Mean values ± SE are shown.
Increased ABA production is not a requirement for stomatal closure during darkness
It has previously been found that dark-induced stomatal closure in roses require an increase in ABA levels, which is a result of ABA released from ABA-GE.22 To examine the requirement for an ABA increase during dark induced stomatal closure the ABA and ABA-GE concentrations was measured both during the light and dark period.
Our results show that there is no difference in the total leaf ABA concentration between plants grown in high and moderate RH (Fig. 6A). Further, the results also show that there was no significant difference in ABA concentration between light and darkness in either of the RH treatments. This shows that there is no absolute requirement of higher ABA levels during darkness to keep the stomata closed. Also, the results show that there is no significant difference in the concentration of the conjugate ABA-GE (Fig. 6B) between light and dark within either treatment or between the treatments. These results show that an ABA increase does not occur during darkness. However, the stomata still close in moderate RH.
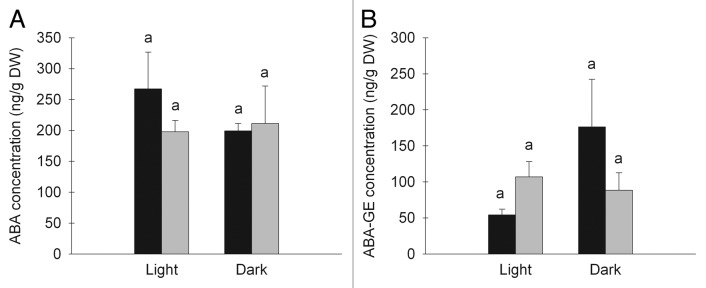
Figure 6. ABA (A) and ABA-GE (B) concentrations in whole leaves of plants grown in moderate (60%; black bars) or high (90%; gray bars) relative air humidity (RH). The concentrations were measured during light and darkness. Different letters indicate significant differences, n = 3. Mean values ± SE are shown.
Discussion
Stomata from plants developed in high RH show weaker response to closing stimuli than those from moderate RH
In this study we found that V. faba developed in continuous high RH have significantly larger stomata compared with plants developed in moderate RH (Fig. 2). This is consistent with previous studies of Rosa x hybrida, A. thaliana and T. virginiana showing that plants developed in continuous high RH have larger stomata.4,5,22,24
We also found that leaves from high RH lost more water during desiccation than those from moderate RH (Fig. 3). This is also consistent with previous studies of A. thaliana, Rosa x hybrida, Begonia x cheimantha, Chrysanthemum morifolium, Euphorbia pulcherrima, Kalanchoe blossfeldiana and T. virginiana.2,4,24,25 Thus, plants developed in continuous high RH have a reduced ability to close the stomata and the reduced stomatal functionality is similar in several different species. It can therefore be concluded that this is a general plant response to high RH, at least in C3 plants.
Darkness is known to induce stomatal closure, although the amount of closure varies between species.1,26 In this study we found that V. faba developed in continuous high RH did not reduce the stomatal aperture in response to darkness. However, V. faba developed in moderate RH reduced the stomatal aperture with about 30% during darkness (Fig. 4A). Similar studies of the diurnal stomatal conductance of intact rose leaves have shown a pattern where plants from both high and moderate RH reduce the stomatal conductance during the dark period.22,27 However, both studies showed a much larger relative change in stomatal conductance in plants developed in moderate RH compared with high RH. Also, studies on A. thaliana and Rosa x hybrida showed that plants developed in moderate RH reduced the stomatal aperture during darkness, while plants developed in high RH did not.4,22
Other stimuli that usually cause stomatal closure are ABA and H2O2.1,20,28 In this study the stomata from both moderate and high RH responded to both ABA and H2O2 by reducing the stomatal aperture (Fig. 4B and C). However, the stomata developed in moderate RH showed a much stronger response to both ABA and H2O2 than those from high RH which showed a very weak response. In a study on T. virginiana it was found that the stomata of plants developed in high RH had reduced response to ABA.24 Similarly, a study on roses showed that ABA-application on fully developed plants grown in high RH had effect on the transpiration rate, but the effect was smaller than in plants developed at moderate RH.27 All these studies show the same trend, where plants developed in high RH have reduced response to ABA. However, the stomata show some response to closing stimuli.
Plants developed in high RH initiate the pathway to stomatal closure in response to ABA, but not in response to darkness
When epidermal peels are placed in the dark they generally produce H2O2 and close the stomata.17 In this study, plants from moderate RH showed an increase in H2O2 levels after transfer to darkness (Fig. 5). However, plants developed in high RH did not increase the H2O2 levels when transferred to the dark (Fig. 5). This shows that darkness induces active stomatal closure through H2O2 production in plants developed in moderate RH, but not in plants developed in high RH. These results are supported by the measurements of stomatal apertures in light and darkness, which show that plants developed in moderate RH close the stomata during darkness, while plants developed in high RH do not (Fig. 4A). The lack of an increased H2O2 production during darkness in plants developed in high RH might therefore be a result of the high RH conditions, which favors open stomata during darkness, overriding the signals from darkness that would otherwise induce stomatal closure. Other studies of A. thaliana, Rosa x hybrida and T. virginiana similarly showed that plants developed in high RH did not close the stomata during darkness.4,22,24 The same studies, including this one, also showed that the ABA levels in leaves developed in high RH did not increase to induce stomatal closure during darkness.4,22 In moderate RH we also found no increase in ABA levels during darkness, but stomatal closure still occurred. Contrary to this, a study on roses showed increased levels of ABA during darkness in moderate RH, due to ABA released from ABA-GE.22 A study on Pseudotsuga menziesii also showed an inverse relationship between ABA and ABA-GE during different growth stages.29 Both these species are woody species. It is possible that ABA regulation through conjugation and subsequent release of ABA from ABA-GE is important in woody species, while other regulation mechanisms are more important in herbaceous species.
One of the most important signals for stomatal closure is ABA.1 The reduced degree of stomatal closure in response to ABA application found in stomata developed in high RH could therefore be a result of reduced sensitivity to ABA. In this study stomata from both high and moderate RH responded to ABA or H2O2 treatments by closing the stomata (Fig. 4B and C). However, the stomata developed in high RH had a very weak response. To investigate if this reduced response is due to a reduced ability to sense ABA and subsequently initiate the pathway toward stomatal closure, the relative amount of the secondary messenger H2O2 was quantified. Plants from both moderate and high RH showed an increase in H2O2 levels after treatment with ABA (Fig. 5). This shows that ABA application initiates the signaling pathway toward stomatal closure in plants developed in both moderate and high RH. However, even though the guard cells in plants developed in high RH produced H2O2 in response to ABA, the amount of stomatal closure was minimal compared with plants from moderate RH (Fig. 4B and C). The reduced stomatal movement in high RH may therefore be caused either by a later step in the ABA dependent-pathway or alternatively by alteration in cell wall structure affecting the guard cell morphology, which could make them physically unable to close properly.30 In a previous study on Commelina communis it has been found that the cell wall composition is important for the stomata to be able to close.30 Lacking arabinan in the cell wall would make them rigid and unable to close.30 It might therefore be hypothesized that high RH decreased guard cell arabinan content, making them unable to move.
Increased leaf level ABA concentration is not necessary for dark induced stomatal closure
It has been shown that H2O2 production is important in both ABA- and dark induced stomatal closure.17 Many studies have showed increased levels of ABA during dark induced stomatal closure.1,22 However the absolute requirement of increased levels of ABA in dark induced stomatal closure is still debatable.
In this study we showed that there was no difference in ABA concentration between light and darkness (Fig. 6). Similarly, there was no difference in ABA-GE concentration between light and darkness. However, there was a clear decrease in stomatal aperture during darkness in plants developed in moderate RH. Under environmental conditions where the plants have high transpiration it is important to close the stomata and preserve water during darkness, when there is no photosynthesis. This stomatal closure has been proposed to require ABA.1 It has also been shown that dark induced stomatal closure requires H2O2 production.17 However, in this study the results for plants grown at moderate RH show that although increased H2O2 levels occur during dark induced stomatal closure, an increase in ABA concentration at leaf levels is not necessarily required. However, further studies are required to fully understand the mechanisms behind dark induced stomatal closure. In roses it has previously been found that the ABA levels increase during darkness, thus closing the stomata.22 Another aspect that needs further studying is the localization of ABA on a cellular level in V. faba. Even though we found no clear relationship between the ABA concentration at leaf level and dark induced stomatal closure in V. faba, the spatial distribution of ABA within the leaf might be important. It has been shown that the ABA levels in the epidermis can be different from the total leaf ABA levels.31 Guard cells can also produce ABA themselves.32 ABA could therefore still play an important part in dark-induced stomatal closure.
Conclusion
This study clearly shows that V. faba plants developed in continuous high RH are able to increase the H2O2 production and thus initiate the pathway toward stomatal closure when treated with ABA. However, they show reduced ability to close the stomata. These results suggest that the reduced stomatal response is caused by a step downstream of H2O2 in the pathway toward stomatal closure. Darkness as a signal for closure did not initiate H2O2 production and subsequent stomatal closure in plants developed at high RH.
We also found that dark induced stomatal closure at moderate RH was independent of total leaf ABA concentration. However, increased H2O2 production still occurred in darkness.
Materials and Methods
Plant material and growth conditions
Vicia faba L. seeds were germinated in 12 cm pots containing peat (L.O.G. Gartnerjord, Rakkested, Norway) and grown in a greenhouse during fall and winter at 20 °C, 80% RH and 20 h supplementary light of 100 µmol m−2 s−1 from high pressure sodium lamps (HPS, Osram NAVT- 400W, Munich, Germany) at the Center for plant research in controlled climate at the Norwegian University of Life Sciences, Ås, Norway (N 59° 40.120', E 10° 46.232'). Plants were grown in the greenhouse for about 1 week, until they were about 10 cm tall.
The plants were then transferred to environmentally controlled growth chambers with moderate RH at 60% (1.05 KPa vpd) or high RH at 92% (0.26 KPa vpd). Both treatments had a temperature of 22 °C, regulated by a PRIVA system (Priva, Ontario, Canada). A photoperiod of 20 h light with a photosynthetic photon flux (PPF) at 400–700 nm of 100 ± 10 µmol m−2 s−1 (LI-COR Light Meter, LI-250 USA) was provided by HPS lamps (Osram NAVT- 400W, Munich, Germany).
Leaflets of the first trifoliate leaf at the onset of flowering were sampled for desiccation test, stomatal size measurements and H2O2 staining.
Response to desiccation
To evaluate the ability of plants to retain water during desiccation one of the leaflets on the first trifoliate leaf was detached and weighted regularly for 3 h. The relative water content at time 0 was set to 100% and the relative water loss at the different time points was calculated. The test was performed in a room with 40% RH, 15 µmol m−2 s−1 irradiance at the surface of the leaves and 22 °C.
Microscopy analysis of stomatal imprints
Epidermal impressions were made of the first trifoliate leaves of plants grown at moderate and high RH, by Suzuki’s Universal Micro-Printing (SUMP) method using SUMP liquid and SUMP plate B (SUMP Laboratory, Tokyo, Japan) as described previously (Tanaka et al. 2005). Imprints were taken in the middle of the light and dark period in both RH treatments. The SUMP imprints were observed under a light microscope (Leitz, Labolux K, Type 0.2, Wetzlar, Germany) and images of stomata were obtained with a Leica camera (Leica DC200, Heerbrugg, Switzerland). Stomatal aperture was measured using Fiji (Fiji Is Just ImageJ, http://fiji.sc/).
Stomatal size and closing ability using epidermal peels
Peels were taken from the lower side of leaflets of the first trifoliate leaves of plants grown at high and moderate RH. The individual plants within each RH treatment were cultivated at separate times. The peels were placed in MES buffer (10 mM MES, 50 mM KCl, 100 µM CaCl2, pH 6.15) for 2.5 h while receiving light from Metal Halide lamps (HPI, MBID250/T/H, Kolorarc, Hungary) at an irradiance of 100 µmol m−2 s−1. First half of the peels from a leaf from 4 replicate plants were moved to another buffer containing 10 µM ABA (10 mM MES, 50 mM KCl, 100 µM CaCl2, 10µM ABA, pH 6.15). In a subsequent experiment half of the peels from each leaf from 4 replicate plants were after 2.5 h of MES buffer moved to a buffer containing 1mM H2O2 (10 mM MES, 50 mM KCl, 100 µM CaCl2, 1mM H2O2, pH 6.15). All peels were then incubated for another 2.5 h receiving light from HPI lamps (MBID250/T/H) of an irradiance of 100 µmol m−2 s−1. Control peels were similarly incubated for another 2.5 h in MES buffer under the same conditions. Images of the stomata from ABA, H2O2 as well as the control treatment were then taken with a Leica camera (Leica DFC 425, Leica Microsystems GmbH, Wetzlar, Germany) connected to a light microscope (Leitz, Labolux K, Wetzlar, Germany). The stomatal aperture was recorded in all images of peels from all treatments, while the pore length was only measured in the light. All measurements were done using the UTHSCSA ImageTool 3.0 (University of Texas Health Science center in San Antonio USA).
H2O2 quantification
Separate MES-buffer-incubated peels (2.5 h) from plants grown at high and moderate RH, all as described above, were cut into squares of 0.5 x 0.5 cm. These were then moved to either a buffer containing 10 µM ABA (as described above), fresh MES buffer which were placed in the dark for 2.5 h or kept in the same MES buffer in light. After 2.5 h the peels from each of the buffer treatments were placed in a Tris buffer (10 mM Tris, 50mM KCl, pH 7.2) containing 50 µM H2DCF-DA (dichlorodihydrofluorescein diacetate) dissolved in dimethyl sulfoxide, for 10 min in the dark. Excess dye was then removed by washing twice with Tris buffer for 1 min. The peels were then photographed through a confocal laser scanning microscope (Leica TCS SP 5 mounted on a Leica DMI 6000 microscope, Wetzlar, Germany), with excitation 488 nm and emission 495–595 nm. Eight images were taken of each peel and all stomata on the images were used in the analysis. The fluorescent images were analyzed using Fiji (Fiji Is Just ImageJ, http://fiji.sc/). The mean fluorescence within the outer edges of the guard cells was used in the statistical analyses.
ABA quantification
Chemicals and Calibration Curves
A number of compounds namely DPA, ABA-GE, PA, 7'-OH-ABA, neoPA, and trans-ABA were synthesized and prepared at the National Research Council of Canada, Saskatoon, SK, Canada. The deuterated forms of the hormones which were used as internal standards that include d3-DPA, d5-ABAGE, d3-PA, d4–7'-OH-ABA, d3-neoPA, d4-ABA and d4-trans-ABA were synthesized and prepared at NRCC SK.33,34 The deuterated forms of selected hormones used as recovery (external) standards, d6-ABA and d2-ABA-GE, were prepared and synthesized at NRCC SK. Calibration curves were created for all compounds of interest.
Instrumentation
Analysis was performed on a UPLC/ESI-MS/MS utilizing a Waters ACQUITY UPLC system, equipped with a binary solvent delivery manager and a sample manager coupled to a Waters Micromass Quattro Premier XE quadrupole tandem mass spectrometer via a Z-spray interface. MassLynx™ and QuanLynx™ (Micromass, Manchester, UK) were used for data acquisition and data analysis.
Extraction and purification
Samples taken during light and darkness were quickly frozen in liquid nitrogen, before they were freeze-dried and homogenized. An aliquot (100 μL) containing the deuterated internal standards, each at a concentration of 0.2 ng μL-1, was added to weighed homogenized plant tissue; 3 mL of isopropanol:water:glacial acetic acid (80:19:1, v/v/v) was then added, and the samples were agitated in the dark for 24 h at 4 °C. Samples were then centrifuged and the supernatant was isolated and dried on a Büchi Syncore Polyvap (Büchi, Switzerland). Samples were reconstituted in 100 μL acidified methanol, adjusted to 1 mL with acidified water, and then partitioned against 2 mL hexane. After 30 min, the aqueous layer was isolated and dried as above. Dry samples were reconstituted in 100 μL acidified methanol and adjusted to 1 mL with acidified water. The reconstituted samples were loaded onto equilibrated Oasis HLB cartridges (Waters, Mississauga, ON, Canada), washed with acidified water, and eluted with acetonitrile: water:glacial acetic acid (30:69:1, v/v/v). The eluate was then dried on a LABCONCO centrivap concentrator (Labconco Corporation, Kansas City, MO USA). An internal standard blank was prepared with 100 μL of the deuterated internal standards mixture. Quality control standards (QC) were prepared by adding 100 μL and 30 μL (separately) of a mixture containing the analytes of interest, each at a concentration of 0.2 ng μL-1, to 100 μL of the internal standard mix. Finally, samples, blanks, and QCs were reconstituted in an aqueous solution of 40% methanol (v/v), containing 0.5% acetic acid and 0.1 ng μL-1 of each of the recovery standards.
Hormone quantification by HPLC-ESI-MS/MS
The samples were subjected to UPLC-ES-MS/MS analysis and quantification.35 Samples were injected onto an ACQUITY UPLC® HSS C18 column (2.1x100 mm, 1.8 μm) with an ACQUITY HSS C18 VanGuard Pre-column (2.1x5 mm, 1.8 μm) and separated by a gradient elution of water containing 0.025% acetic acid against an increasing percentage of acetonitrile containing 0.025% acetic acid. Briefly, the analysis utilizes the Multiple Reaction Monitoring (MRM) function of the MassLynx v4.1 (Waters Inc) control software. The resulting chromatographic traces are quantified off-line by the QuanLynx v4.1 software (Waters Inc) wherein each trace is integrated and the resulting ratio of signals (non-deuterated/internal standard) is compared with a previously constructed calibration curve to yield the amount of analyte present (ng per sample). Calibration curves were generated from the MRM signals obtained from standard solutions based on the ratio of the chromatographic peak area for each analyte to that of the corresponding internal standard.35 The QC samples, internal standard blanks and solvent blanks were also prepared and analyzed along each batch of tissue samples.
Statistical analysis
The results were analyzed using normally distributed general linear model (GLM) and the Tukey test. Differences with P ≤ 0.050 were considered significantly different. All statistical analyses were performed in Minitab 16.1.1 (Minitab 16.1.1, windows version, State College, Pennsylvania USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Tallman G. Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J Exp Bot. 2004;55:1963–76. doi: 10.1093/jxb/erh212. [DOI] [PubMed] [Google Scholar]
- 2.Torre S, Fjeld T. Water loss and postharvest characteristics of cut roses grown at high or moderate relative air humidity. Sci Hortic (Amsterdam) 2001;89:217–26. doi: 10.1016/S0304-4238(00)00229-6. [DOI] [Google Scholar]
- 3.Fanourakis D, Carvalho SMP, Almeida DPF, Heuvelink E. Avoiding high relative air humidity during critical stages of leaf ontogeny is decisive for stomatal functioning. Physiol Plant. 2011;142:274–86. doi: 10.1111/j.1399-3054.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 4.Arve LE. Stomatal functioning and abscisic acid (ABA) regulation in plants developed in different air humidity regimes. Ås: Norwegian University of Life Sciences, 2013. [Google Scholar]
- 5.Torre S, Fjeld T, Gislerød HR, Moe R. Leaf anatomy and stomatal morphology of greenhouse roses grown at moderate or high air humidity. J Am Soc Hortic Sci. 2003;128:598–602. [Google Scholar]
- 6.Santamaria JM, Davies WJ, Atkinson CJ. Stomata of micropropagated Delphinium plants respond to ABA, CO2, light and water potential, but fail to close fully. J Exp Bot. 1993;44:99–107. doi: 10.1093/jxb/44.1.99. [DOI] [Google Scholar]
- 7.Fordham MC, Harrison-Murray RS, Knight L, Evered CE. Effects of leaf wetting and high humidity on stomatal function in leafy cuttings and intact plants of Corylus maxima. Physiol Plant. 2001;113:233–40. doi: 10.1034/j.1399-3054.2001.1130211.x. [DOI] [PubMed] [Google Scholar]
- 8.Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008;147:1984–93. doi: 10.1104/pp.108.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB. Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol. 2000;42:833–45. doi: 10.1023/A:1006448428401. [DOI] [PubMed] [Google Scholar]
- 10.Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plant Sci. 1999;4:472–8. doi: 10.1016/S1360-1385(99)01497-1. [DOI] [PubMed] [Google Scholar]
- 11.Priest DM, Ambrose SJ, Vaistij FE, Elias L, Higgins GS, Ross ARS, Abrams SR, Bowles DJ. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006;46:492–502. doi: 10.1111/j.1365-313X.2006.02701.x. [DOI] [PubMed] [Google Scholar]
- 12.Nejad AR, van Meeteren U. The role of abscisic acid in disturbed stomatal response characteristics of Tradescantia virginiana during growth at high relative air humidity. J Exp Bot. 2007;58:627–36. doi: 10.1093/jxb/erl234. [DOI] [PubMed] [Google Scholar]
- 13.Giday H, Fanourakis D, Kjaer KH, Fomsgaard IS, Ottosen CO. Foliar abscisic acid content underlies genotypic variation in stomatal responsiveness after growth at high relative air humidity. Ann Bot. 2013;112:1857–67. doi: 10.1093/aob/mct220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliniaeifard S, van Meeteren U. Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? J Exp Bot. 2013;64:3551–66. doi: 10.1093/jxb/ert192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell. 2012;24:2483–96. doi: 10.1105/tpc.112.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi-Saha A, Valon C, Leung J. A brand new START: abscisic acid perception and transduction in the guard cell. Sci Signal. 2011;4:re4. doi: 10.1126/scisignal.2002164. [DOI] [PubMed] [Google Scholar]
- 17.Desikan R, Cheung MK, Clarke A, Golding S, Sagi M, Fluhr R, Rock C, Hancock J, Neill S. Hydrogen peroxide is a common signal for darkness- and ABA-induced stomatal closure in Pisum sativum. Funct Plant Biol. 2004;31:913–20. doi: 10.1071/FP04035. [DOI] [PubMed] [Google Scholar]
- 18.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–22. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 19.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. 2008:165-76 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–48. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan LM, Zhao Z, Assmann SM. Guard cells: a dynamic signaling model. Curr Opin Plant Biol. 2004;7:537–46. doi: 10.1016/j.pbi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Arve LE, Terfa MT, Gislerød HR, Olsen JE, Torre S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013;36:382–92. doi: 10.1111/j.1365-3040.2012.02580.x. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds-Henne CE, Langenegger A, Mani J, Schenk N, Zumsteg A, Feller U. Interactions between temperature, drought and stomatal opening in legumes. Environ Exp Bot. 2010;68:37–43. doi: 10.1016/j.envexpbot.2009.11.002. [DOI] [Google Scholar]
- 24.Nejad AR, van Meeteren U. Stomatal response characteristics of Tradescantia virginiana grown at high relative air humidity. Physiol Plant. 2005;125:324–32. doi: 10.1111/j.1399-3054.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen LM. Effects of air humidity on growth, flowering, keeping quality and water relations of four short-day greenhouse species. Sci Hortic (Amsterdam) 2000;86:299–310. doi: 10.1016/S0304-4238(00)00155-2. [DOI] [Google Scholar]
- 26.Caird MA, Richards JH, Donovan LA. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007;143:4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanourakis D, Carvalho SMP, Almeida DPF, van Kooten O, van Doorn WG, Heuvelink E. Postharvest water relations in cut rose cultivars with contrasting sensitivity to high relative air humidity during growth. Postharvest Biol Technol. 2012;64:64–73. doi: 10.1016/j.postharvbio.2011.09.016. [DOI] [Google Scholar]
- 28.Seo M, Koshiba T. Transport of ABA from the site of biosynthesis to the site of action. J Plant Res. 2011;124:501–7. doi: 10.1007/s10265-011-0411-4. [DOI] [PubMed] [Google Scholar]
- 29.Kong L, Abrams SR, Owen SJ, Van Niejenhuis A, Von Aderkas P. Dynamic changes in concentrations of auxin, cytokinin, ABA and selected metabolites in multiple genotypes of Douglas-fir (Pseudotsuga menziesii) during a growing season. Tree Physiol. 2009;29:183–90. doi: 10.1093/treephys/tpn009. [DOI] [PubMed] [Google Scholar]
- 30.Jones L, Milne JL, Ashford D, McQueen-Mason SJ. Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci U S A. 2003;100:11783–8. doi: 10.1073/pnas.1832434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 32.Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol. 2013;23:53–7. doi: 10.1016/j.cub.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Abrams SR, Nelson K, Ambrose SJ. Deuterated abscisic acid analogs for mass spectrometry and metabolism studies. J Labelled Comp Radiopharm. 2003;46:273–83. doi: 10.1002/jlcr.670. [DOI] [Google Scholar]
- 34.Zaharia LI, Galka MM, Ambrose SJ, Abrams SR. Preparation of deuterated abscisic acid metabolites for use in mass spectrometry and feeding studies. J Labelled Comp Radiopharm. 2005;48:435–45. doi: 10.1002/jlcr.939. [DOI] [Google Scholar]
- 35.Ross ARS, Ambrose SJ, Cutler AJ, Feurtado JA, Kermode AR, Nelson K, Zhou R, Abrams SR. Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry with multiple reaction monitoring. Anal Biochem. 2004;329:324–33. doi: 10.1016/j.ab.2004.02.026. [DOI] [PubMed] [Google Scholar]