Abstract
The ability of animals to propel themselves efficiently through a fluid medium is ecologically advantageous. Flexible components that influence vortex interactions are widespread among animal propulsors. However the mechanisms by which vortices are enhanced and appropriately positioned for thrust generation are still poorly understood. Here, we describe how kinematic propulsor movements of a jellyfish can enhance and reposition a vortex ring that allows the recapture of wake energy for secondary thrust generation and efficient locomotion. We use high-speed video and digital particle image velocimetry (DPIV) to resolve kinematics simultaneously with fluid structures. These results provide new insight into how animals can manipulate fluid structures to reduce metabolic energy demands of swimming muscles and may have implications in bio-inspired design.
Keywords: energetics, fluid dynamics, kinematics, swimming efficiency, vortex
Energy saving mechanisms during locomotion are widespread in the animal kingdom. Elastic properties in tissues reduce the energetic expenditure by flying animals,1,2 runners,3,4 and swimmers.5 Animals can also obtain energy savings from properties of the surrounding fluid. For example, birds are known to reduce energy expenditure by flying in formation which reduces drag6-8 and fish exhibit similar energy savings when swimming in a school.9 Individuals can also benefit by creating and favorably positioning vortices relative to the body.10-12 Thus, energetically efficient propulsion is important for motile organisms and should be positively selected for in nature. One reason for this is that energy consumed by swimming can reduce reproductive output,13 so it stands to reason that energetically efficient locomotion is an important evolutionary driver. In the case of jellyfish, energetically efficient locomotion may also allow populations to persist during periods of low food availability or invade a niche where food can be patchy or highly dilute.
Vortex formation and enhancement is crucial for generating efficient locomotion.10,14-16 Fish are a successful group and are well adapted for using their flexible fins and bodies to enhance the vorticity of fluid around propulsive structures.17 The result is that fish swimming exhibits a lower cost-of-transport (COT) than nearly all other locomotive mechanisms.18,19 In other words, fish use comparatively little energy to get from point A to point B. However, a group of animals not typically associated with efficient propulsion takes vortex formation and utilization to the next level. Jellyfish have been traditionally viewed as inefficient swimmers,20 primarily due to their slow swimming speeds. After all, most cannot even swim against modest ocean currents. However, what they lack in speed, they more than make up for in energy savings through a unique mechanism of recapturing vortex energy resulting in exceptionally low COT.21,22 The moon jellyfish (Aurelia aurita) swims by contracting and relaxing its flexible bell. The most obvious fluid structure created is the formation of a vortex ring (starting vortex) in the wake behind the animal.23 However, a second, less conspicuous vortex ring (stopping vortex) with opposite spin vorticity, contributes greatly to the high energetic efficiency of jellyfish propulsion. This second vortex initially forms on the outside of the body, just upstream of the starting vortex (Fig. 1). One of the real advantages of jellyfish propulsion is the ability to enhance the circulation of this stopping vortex and reposition it underneath the animal at the subumbrellar surface in order to obtain a secondary boost in velocity.24 While the importance of the stopping vortex to swimming with a low COT is now recognized, the details of how the stopping vortex, which originates midway up the body on the exumbrellar surface, comes to reside at the animal’s subumbrellar surface and exhibits high vorticity remains undescribed. In this manuscript we visualize and describe this mechanism used by jellyfish to enhance and reposition the stopping vortex in such a manner so as to gain a significant propulsive boost using little (if any) additional energy.
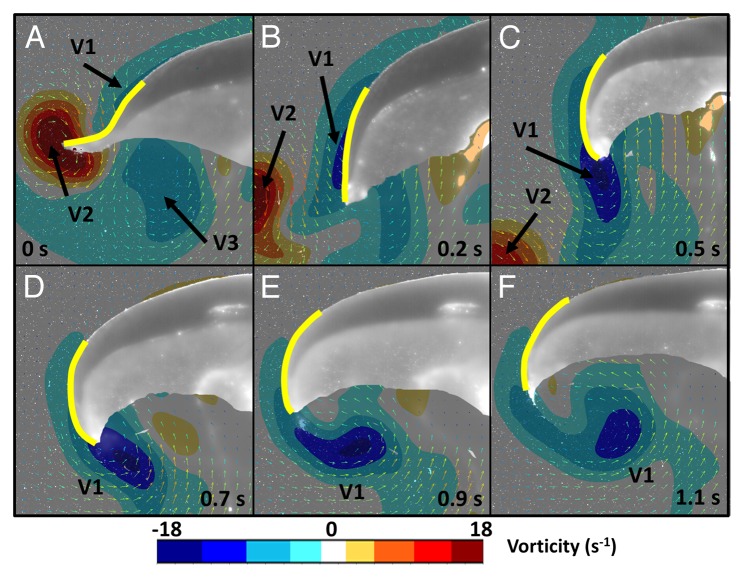
Figure 1. Partial view of a 4 cm jellyfish (Aurelia aurtia) illustrating conformational changes of the bell margin (yellow) and resulting control/enhancement of vortex rings. (A) Early stage of contraction where substantial outward bending occurs and strength of the starting vortex (V2) increases and interacts with the previous swimming cycle’s stopping vortex (V3). The current cycle’s stopping vortex (V1) forms upstream of V2 but has low vorticity. (B) Near the end of the contraction cycle bending is reduced and the stopping vortex (V1) is now positioned near the bell margin. (C) The relaxation phase begins and the bell margin exhibits inward bending which coincides with enhancement of V1 and repositioning at the tip of the bell margin. (D-F) Expansion of the bell continues upwards and outwards as bending becomes reduced. This motion aids in positioning the vortex (V1) under the bell and increasing the vorticity.
During the initial stages of contraction, vorticity, and circulation of the starting vortex increases rapidly and is aided by inward bending at the bell margin16 (Fig. 1A). Comparatively, at this stage the stopping vortex is smaller, exhibits lower vorticity and is located upstream of the starting vortex. Peak vorticity of starting and stopping vortices are 26 s−1 and 5 s−1, respectively. As the contraction progresses, the animal accelerates forward and the starting vortex separates from the body. As this occurs, the stopping vortex also moves relative to the animal, toward the bell margin and peak vorticity increases to 12 s−1 (Fig. 1B). During this period (nearing the end of contraction) there is a substantial change in the kinematics at the bell margin. Bending in this region becomes greatly reduced (Fig. 1C). The inward inflexion which aids in enhancing vorticity of the starting vortex is presumably no longer required because the starting vortex has now separated from the body. Also, the kinematics responsible for producing a strong starting vortex contributes very little to the strength of the stopping vortex as it remains comparatively weak. Completion of the contraction phase of the swimming cycle involves reduction of inward bending at the bell margin and gives way to an outward bend that positions the tip of the margin toward the center of the animal (Fig. 2). This acts to increase the vorticity of the stopping vortex to 20 s−1 and also position it below the animal at the completion of the contraction. However, at this stage the stopping vortex is still associated with the exumbrellar (outer) surface of the jellyfish at the bell margin (Fig. 1C).
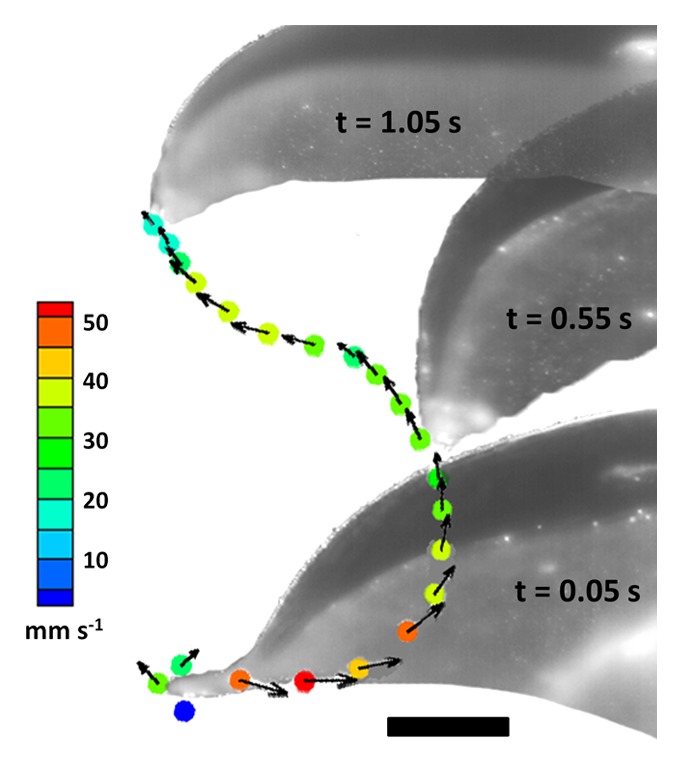
Figure 2. Motion of the bell margin through a complete swimming cycle. Time intervals between points is 0.05 s. Scale bar = 5 mm.
In order to obtain positive thrust from this stopping vortex, it must be positioned underneath the animal (at the subumbrellar surface). Additional kinematic motion of the bell margin during the relaxation phase of the swimming cycle is important to accomplish this. Therefore the relaxation phase becomes much more than simply resetting for the next contraction. During relaxation the bell re-expands outwards and upwards (Fig. 1D-F; Figure 2). Velocity at the bell margin is slower during the relaxation phase with a maximum speed of 38 mm s−1 compared with 52 mm s−1 during the contraction. The outward motion and reduction in bending combine in a constructive manner relative to the vortex rotation, thereby increasing its vorticity to 22 s−1. This increase in vortex strength ultimately leads to greater induced flow against the subumbrellar surface and localized high pressure, resulting in significant thrust. It is important to note that while interactions of the starting vortex with the previous swimming cycle’s stopping vortex may be important for thrust generation and direction, the secondary boost in velocity the jellyfish receives from the current swimming cycle’s stopping vortex does not appear to involve interaction with any other vortices.
While these results aid our understanding of the processes involved in secondary thrust generation in jellyfish swimming, there are questions that remain unresolved. For instance, do these animals rely on feedback or sensing mechanisms to coordinate kinematics that control vortices for optimal efficiency? This becomes important when considering Aurelia aurita are ectothermic animals that naturally occur at a large range of temperatures. Colder temperatures may alter the contraction rate and speed of the bell which can influence vortex formation and strength. Cooler water temperatures also exhibit higher viscosity and a change in temperature from 30 to 10 °C results in a 37% increase in kinematic viscosity.24 Naturally occurring changes in viscosity, independent of temperature, can also be substantial with increases of up to 259% observed during blooms of Phaeocystis globosa.25 Elevated viscosity will result in faster dissipation of vortices which can feasibly reduce effectiveness of the energy recapture mechanism from the stopping vortex. Thus, it stands to reason that kinematics such as; bell speed, inflexion and contraction rate may require fine scale regulation and feedback control to maintain optimal propulsive efficiency at different temperatures.
Swimming contractions are initiated by rhopalial pacemakers at the bell margin associated with the subumbrellar nerve net.26 These structures also contain statocysts and touch plates, a group of sensory cells, responsible for sensing movement and orientation.27 These organs in scyphomedusae are capable of maintaining an orientation and can compensate when they are steered off course.28 Thus, the framework exists for sensing/regulation and feedback of kinematics in response to acceleration. If changes in viscosity alter stopping vortex dissipation rate and strength, the amount of time the medusae will gain sufficient thrust will also change and the animal should decelerate more quickly. If this can be sensed by rhopalia a signal could be initiated to begin contractions earlier and/or bell kinematics adjusted (i.e., contraction speed, bell margin inflexion) to maintain vortex strength. However, additional studies will be required to elucidate these questions.
The role of enhancing and positioning vortices is critical to efficient locomotion. For example, cores of vortices have low pressure and are known to aid in propulsion and lift generation.29 Therefore, mechanisms which enhance these vortices or control the position relative to the propulsive structure are important to understand from both an ecological perspective as well as from a design perspective. Jellyfish provide a useful model for investigation of animal locomotion due to their simple body plans which allows the assumption of axisymmetry during straight swimming. Axisymmetry permits extension of 2-dimensional methods to reasonable 3-dimensional estimates. Most animal locomotion however, involves complex kinematics and body plans that deviate significantly from axisymmetry. Therefore, knowing how vortices interact with body surfaces and with each other in 3 dimensions will be important for future studies.
Materials and Methods
Jellyfish (Aurelia aurita) were obtained from the New England Aquarium and maintained in 20-L aquaria at 20 °C. For recording, individual animals were placed into a glass filming vessel (30 × 10 × 25 cm) filled with filtered seawater. Recordings of free-swimming animals were acquired by a high-speed digital video camera (Fastcam 1024 PCI; Photron) at 1,000 frames per second. Detailed swimming kinematics (2D) were obtained using ImageJ v1.46 software (National Institutes of Health) to track the x and y coordinates of the apex of the jellyfish bell margin over time. Bell margin speed was calculated from the change in the position of the margin tip over time as:
Jellyfish were illuminated with a laser sheet (680 nm, 2W continuous wave; LaVision) oriented perpendicular to the camera’s optical axis to provide a distinctive body outline for image analysis and to ensure the animal remained in-plane, which ensures the accuracy of 2D estimates of position and velocity.
Fluid motion created by the jellyfish while swimming was quantified using 2D digital particle image velocimetry (DPIV). Using the setup described in Gemmell et al. 2013, the filtered seawater was seeded with 10-μm hollow glass beads. The velocities of particles illuminated in the laser sheet were determined from sequential images analyzed using a cross correlation algorithm (LaVision software). Image pairs were analyzed with shifting, overlapping interrogation windows of a decreasing size of 64 × 64 pixels to 32 × 32 pixels.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the New England Aquarium for providing cultured medusae. B.J.G., J.H.C., and S.P.C. were supported by a Multidisciplinary University Research Initiative (MURI) Grant N00014–08–1-0654 through the Office of Naval Research (ONR).
References
- 1.Dickinson MH, Lighton JR. Muscle efficiency and elastic storage in the flight motor of Drosophila. Science. 1995;268:87–90. doi: 10.1126/science.7701346. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114–7. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- 3.Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–81. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asmussen E, Bonde-Petersen F. Apparent efficiency and storage of elastic energy in human muscles during exercise. Acta Physiol Scand. 1974;92:537–45. doi: 10.1111/j.1748-1716.1974.tb05776.x. [DOI] [PubMed] [Google Scholar]
- 5.Blickhan R, Cheng J-Y. Energy storage by elastic mechanisms in the tail of large swimmers—a re-evaluation. J Theor Biol. 1994;168:315–21. doi: 10.1006/jtbi.1994.1112. [DOI] [Google Scholar]
- 6.Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. Energy saving in flight formation. Nature. 2001;413:697–8. doi: 10.1038/35099670. [DOI] [PubMed] [Google Scholar]
- 7.Cutts C, Speakman J. Energy savings in formation flight of pink-footed geese. J Exp Biol. 1994;189:251–61. doi: 10.1242/jeb.189.1.251. [DOI] [PubMed] [Google Scholar]
- 8.Lissaman PB, Shollenberger CA. Formation flight of birds. Science. 1970;168:1003–5. doi: 10.1126/science.168.3934.1003. [DOI] [PubMed] [Google Scholar]
- 9.Herskin J, Steffensen J. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J Fish Biol. 1998;53:366–76. doi: 10.1111/j.1095-8649.1998.tb00986.x. [DOI] [Google Scholar]
- 10.Streitlien K, Triantafyllou GS, Triantafyllou MS. Efficient foil propulsion through vortex control. AIAA J. 1996;34:2315–9. doi: 10.2514/3.13396. [DOI] [Google Scholar]
- 11.Anderson J, Streitlien K, Barrett D, Triantafyllou M. Oscillating foils of high propulsive efficiency. J Fluid Mech. 1998;360:41–72. doi: 10.1017/S0022112097008392. [DOI] [Google Scholar]
- 12.Liao JC, Beal DN, Lauder GV, Triantafyllou MS. The Kármán gait: novel body kinematics of rainbow trout swimming in a vortex street. J Exp Biol. 2003;206:1059–73. doi: 10.1242/jeb.00209. [DOI] [PubMed] [Google Scholar]
- 13.Koch F, Wieser W. Partitioning of energy in fish: Can reduction of swimming activity compensate for the cost of production? J Exp Biol. 1983;107:141–6. [Google Scholar]
- 14.Heathcote S, Wang Z, Gursul I. Effect of spanwise flexibility on flapping wing propulsion. J Fluids Structures. 2008;24:183–99. doi: 10.1016/j.jfluidstructs.2007.08.003. [DOI] [Google Scholar]
- 15.Taylor GS, Gursul I. Lift enhancement over a flexible delta wing. 2nd AIAA Flow Control Conference, Portland, OR, AIAA Paper, 2004. [Google Scholar]
- 16.Colin SP, Costello JH, Dabiri JO, Villanueva A, Blottman JB, Gemmell BJ, Priya S. Biomimetic and live medusae reveal the mechanistic advantages of a flexible bell margin. PLoS One. 2012;7:e48909. doi: 10.1371/journal.pone.0048909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauder GV, Madden PG. Fish locomotion: kinematics and hydrodynamics of flexible foil-like fins. Exp Fluids. 2007;43:641–53. doi: 10.1007/s00348-007-0357-4. [DOI] [Google Scholar]
- 18.Schmidt-Nielsen K. Locomotion: energy cost of swimming, flying, and running. Science. 1972;177:222–8. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 19.Tucker VA. The energetic cost of moving about. Am Sci. 1975;63:413–9. [PubMed] [Google Scholar]
- 20.Dabiri JO, Colin SP, Katija K, Costello JH. A wake-based correlate of swimming performance and foraging behavior in seven co-occurring jellyfish species. J Exp Biol. 2010;213:1217–25. doi: 10.1242/jeb.034660. [DOI] [PubMed] [Google Scholar]
- 21.Larson R. Costs of transport for the scyphomedusa Stomolophus meleagris L. Agassiz. Can J Zool. 1987;65:2690–5. doi: 10.1139/z87-408. [DOI] [Google Scholar]
- 22.Gemmell BJ, Costello JH, Colin SP, Stewart CJ, Dabiri JO, Tafti D, Priya S. Passive energy recapture in jellyfish contributes to propulsive advantage over other metazoans. Proc Natl Acad Sci U S A. 2013;110:17904–9. doi: 10.1073/pnas.1306983110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabiri JO, Colin SP, Costello JH, Gharib M. Flow patterns generated by oblate medusan jellyfish: field measurements and laboratory analyses. J Exp Biol. 2005;208:1257–65. doi: 10.1242/jeb.01519. [DOI] [PubMed] [Google Scholar]
- 24.Gemmell BJ, Sheng J, Buskey EJ. Compensatory escape mechanism at low Reynolds number. Proc Natl Acad Sci U S A. 2013;110:4661–6. doi: 10.1073/pnas.1212148110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seuront L, Vincent D, Mitchell JG. Biologically induced modification of seawater viscosity in the Eastern English Channel during a< i> Phaeocystis globosa</i> spring bloom. J Mar Syst. 2006;61:118–33. doi: 10.1016/j.jmarsys.2005.04.010. [DOI] [Google Scholar]
- 26.Satterlie R, Spencer A. Swimming control in a cubomedusan jellyfish. 1979 [Google Scholar]
- 27.Hündgen M, Biela C. Fine structure of touch-plates in the scyphomedusan Aurelia aurita. J Ultrastruct Res. 1982;80:178–84. doi: 10.1016/S0022-5320(82)90016-8. [DOI] [PubMed] [Google Scholar]
- 28.Shanks AL, Graham WM. Orientated swimming in the jellyfish< i> Stomolopus meleagris</i> L. Agassiz (Scyphozoan: Rhizostomida) J Exp Mar Biol Ecol. 1987;108:159–69. doi: 10.1016/S0022-0981(87)80020-5. [DOI] [Google Scholar]
- 29.Shyy W, Liu H. Flapping wings and aerodynamic lift: the role of leading-edge vortices. AIAA J. 2007;45:2817–9. doi: 10.2514/1.33205. [DOI] [Google Scholar]