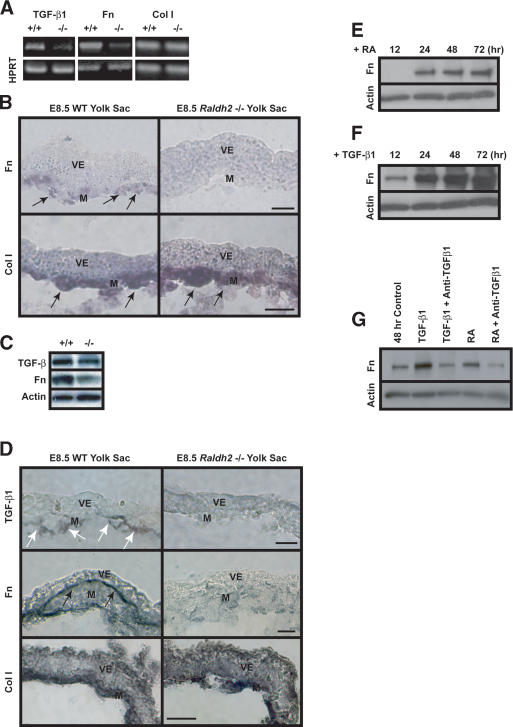
Figure 2.
RA regulation of TGF-β1 and Fn in endothelial cells. (A) sqRT-PCR revealed decreased expression of TGF-β1 and Fn transcripts in E8.5 Raldh2-/- yolk sacs compared with wild type (WT). Col I transcript levels were similar in both Raldh2-/- and wild-type yolk sacs; HPRT was used as an internal control. (B) In situ hybridization of E8.5 yolk sac sections revealed that Fn (top) and Col I (bottom) transcripts were expressed in the mesoderm (arrows); however, Fn transcript levels were down-regulated in Raldh2-/- (right) compared with wild type (WT; left). Bar, 25 μm. (C) Western blot analyses of E8.5 Raldh2-/- mutant and wild-type (WT) yolk sacs demonstrated decreased levels of TGF-β1 and Fn protein. Actin was used as a loading control. (D) Immunohistochemistry of E8.5 yolk sac sections revealed that levels of TGF-β1 protein (top row), specifically expressed in endothelial cells (arrows), and Fn protein (middle row), deposited between the visceral endoderm and mesoderm (black arrows), were decreased in Raldh2-/- yolk sac (right) compared with wild type (WT; left). Protein levels of Col I (bottom row) were similar in Raldh2-/- and wild type. Bar, 25 μm. (E-G) Bovine aortic endothelial cells were cultured for up to 72 h in the presence of 1 μM RA (D) or 1 ng/mL TGF-β1 (F). Western blot demonstrated that RA increased Fn production over control levels at 24 h (E,G). TGF-β1 significantly increased Fn production in endothelial cells starting at 12 h (F,G). (G) Neutralizing anti-sera against TGF-β1, TGF-β2, and TGF-β3 suppressed RA-induced Fn production in endothelial cells, as well as TGF-β1-induced Fn production. (M) Mesoderm; (V) visceral endoderm.