Figure 3.
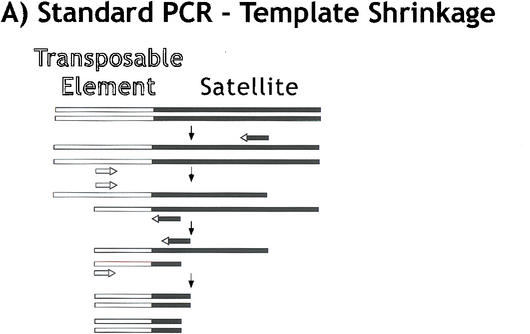
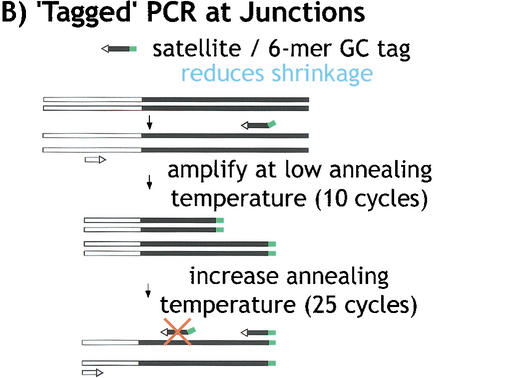
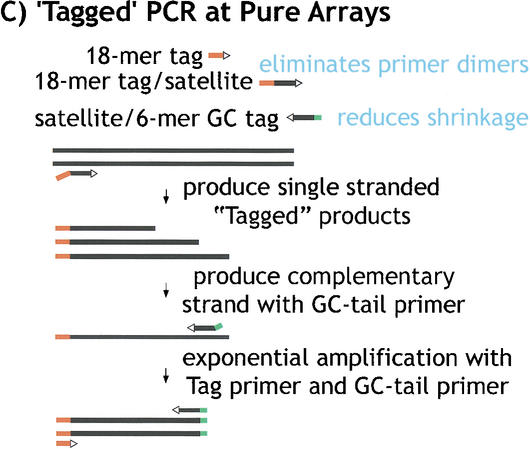
“Tagged” polymerase chain reaction (PCR) methods for cloning and sequencing heterochromatin. (A) Standard PCR amplifications with one satellite primer and one “unique” primer (homologous to the end of the transposon) result in shrinkage with each round of amplification. A satellite repeat primer will anneal anywhere in the repeated template, not just at the ends, and thus successive rounds of amplification will result in shorter and shorter products. The template shrinkage problem is even greater when two satellite primers are used to amplify pure satellite arrays (not shown), and in addition, the self-complementarity of the two satellite primers results in primer-dimer formation (not shown). (B) Junctions between complex DNA (transposons in this case) and satellite arrays are successfully amplified with significantly less shrinkage when a hybrid GC-tagged/satellite primer is used. Initial amplification at low stringency incorporates the tag at random within the satellite array then extends across the location of the TE primer; the subsequent exponential amplification reduces shrinkage because high stringency mandates annealing of the entire hybrid primer. This method was also used to generate sequence from bacterial transposon insertions into gel-purified γ1230; in this case, the “complex” primer was homologous to the end of the bacterial transposon. (C) Pure satellite arrays were amplified with two tagged primers, plus a primer corresponding to one of the tags. One tagged primer was used for single-stranded extension at low stringency; after gel isolation of the single-strand products, the second primer was used to synthesize the complementary strand, then the tag and one tagged primer were used for high stringency amplification.