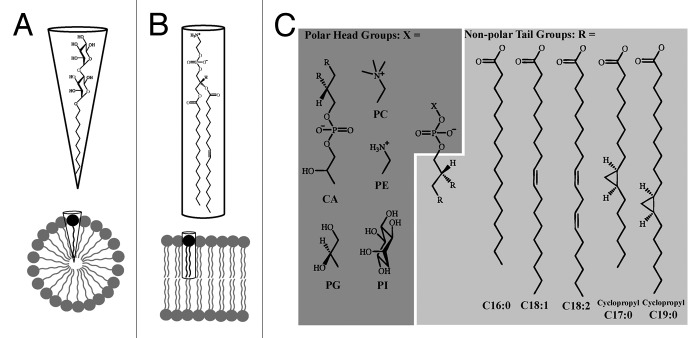
Figure 1. Chemical properties of detergents and lipids. (A) n-Decyl-β-D- maltopyranoside is a common non-ionic detergent used to stabilize TM proteins.5 The large polar head group and relatively short non-polar tail give the detergent a roughly conical shape, which facilitates the formation of detergent micelles. (B) Phosphatidylethanolamine can represent up to 70% of lipid in bacterial membranes;35,46 its roughly cylindrical shape enables the formation of lipid bilayers. (C) In addition to triglycerides and hopanoids, phospholipids are an important component of lipid bilayers. Phospholipids contain three components attached to a glycerol backbone: two non-polar tails (R, light gray) and one polar head group (X, dark gray). Here we show a selection of head groups including cardiolipin (CA), phosphatidylglycerol (PG), phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI); and major tail groups from E. coli: palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2), 9,10-methylene-hexadecanoic acid (cyclopropyl C17:0), and lactobacillic acid (cyclopropyl C19:0). Chemical structures were prepared using ChemDraw (CambridgeSoft Inc.).
