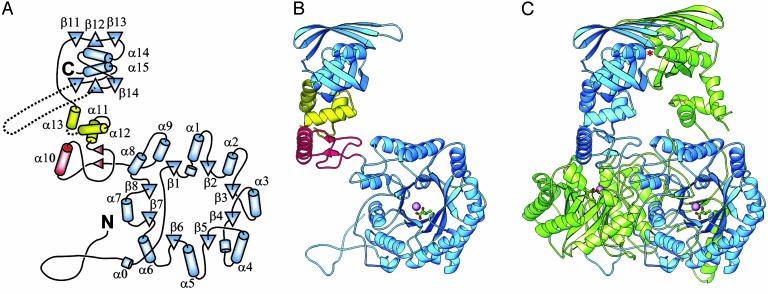
Fig. 1.
Structure of M. tuberculosis α-IPMS. (A) Topology of the monomer. β-Strands are shown as triangles, and helices are shown as cylinders. The helix numbering follows standard practice for TIM barrel domains. The barrel and regulatory domains are colored blue, and subdomains I and II of the linker are colored red and yellow, respectively. (B) Ribbon diagram of the monomer. The bound Zn2+ ion in the catalytic domain is shown as a large magenta sphere, and the α-KIV substrate is depicted as a ball-and-stick model. (C) Ribbon diagram of the dimer. The individual monomers are colored blue and green. An asterisk marks one leucine regulatory binding site (the other is obscured). B and C were drawn with ribbons (26).