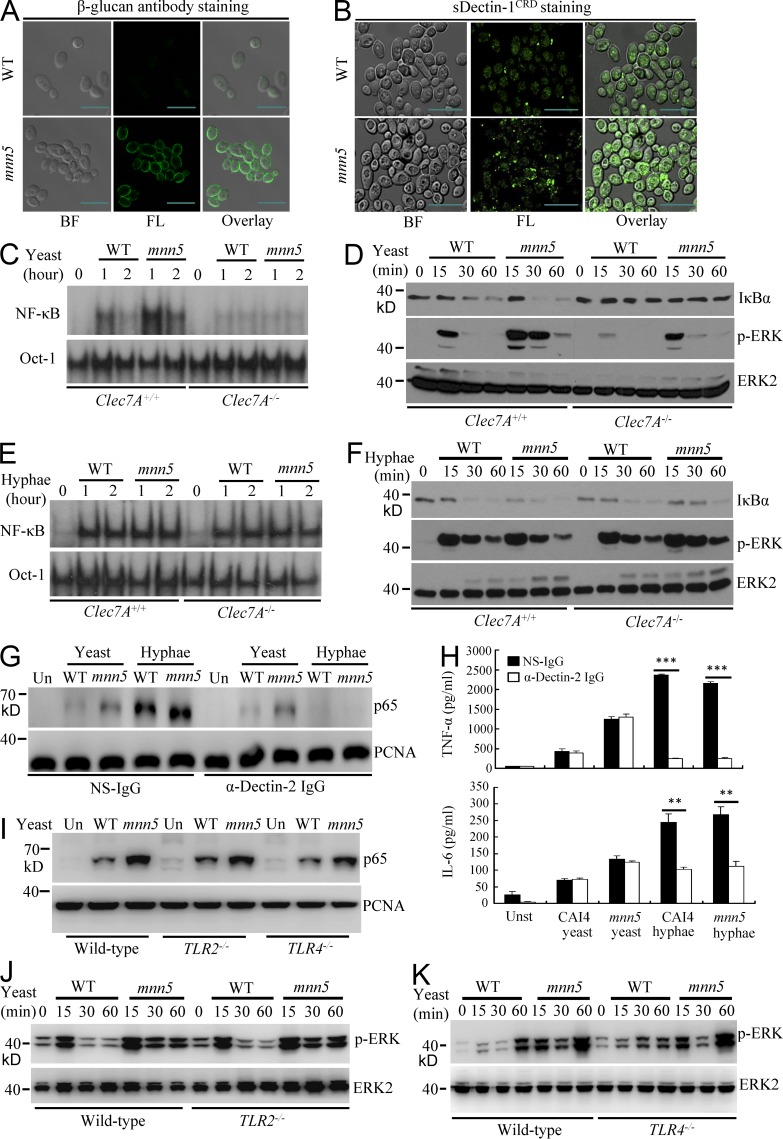
Figure 1.
Surface β-glucans on C. albicans yeasts activate NF-κB and ERK through Dectin-1. (A and B) Surface β-glucan accessibility on WT or mnn5 yeast C. albicans strains, which were stained with anti–β-1,3-glucan monoclonal antibodies (β-glucan), followed by staining with FITC-labeled secondary antibodies (A) or soluble GFP-tagged Dectin-1 carbohydrate recognition domain (sDectin-1CRD; B). Bright field (BF), fluorescence (FL), and overlay (right) are shown individually. Bars, 10 µm. (C and D) WT and Dectin-1 (Clec7A)–deficient BMDMs were stimulated with UV-inactivated WT yeasts or mnn5 yeasts (MOI = 5) for indicated times. Nuclear extracts were subjected to EMSA using 32P-labeled NF-κB or Oct-1 probe (C). Cell lysates were subjected to immunoblotting analysis using the indicated antibodies (D). (E and F) WT and Dectin-1 (Clec7A)–deficient BMDMs were stimulated with hyphae of WT yeasts or mnn5 (MOI = 1) for the indicated times. Nuclear extracts were subjected to EMSA using 32P-labeled NF-κB or Oct-1 probe (E). Cell lysates were subjected to immunoblotting analysis using the indicated antibodies (F). (G) WT BMDMs were stimulated with C. albicans WT or mnn5 yeast (MOI = 5) or hyphae (MOI = 1) in the absence or presence of blocking antibodies against Dectin-2 (α-Dectin-2, 20 µg/ml) for 60 min. Nuclear extracts were prepared from these cells and subjected to immunoblotting analysis using indicated antibodies. (H) WT BMDMs were stimulated with the UV-inactivated yeast (MOI = 5) or hyphae (MOI = 1) form of CAI4 or mnn5 mutant in the absence or presence of blocking antibodies against Dectin-2 (20 µg/ml) for 6 h. The amount of TNF and IL-6 in the cultured media was determined using ELISA. **, P < 0.01; ***, P < 0.001. Data are means ± SD of triplicate wells and are representative of three independent experiments. (I–K) WT, TLR2-, and TRL4-deficient BMDMs were stimulated with UV-inactivated WT (CAI4) or mnn5 strains of C. albicans yeast cells (MOI = 5) for 1 h for preparing nuclear extracts (I) or for the indicated times for preparing cell lysates (J and K). Samples were subjected to immunoblotting analysis using indicated antibodies. Data shown are representative of three independent and reproducible experiments.