Abstract
Objective:
To examine the potential role of 6-sulfo LacNAc+ (slan) dendritic cells (DCs) displaying pronounced proinflammatory properties in the pathogenesis of multiple sclerosis (MS).
Methods:
We determined the presence of slanDCs in demyelinated brain lesions and CSF samples of patients with MS. In addition, we explored the impact of methylprednisolone, interferon-β, glatiramer acetate, or natalizumab on the frequency of blood-circulating slanDCs in patients with MS. We also evaluated whether interferon-β modulates important proinflammatory capabilities of slanDCs.
Results:
SlanDCs accumulate in highly inflammatory brain lesions and are present in the majority of CSF samples of patients with MS. Short-term methylprednisolone administration reduces the percentage of slanDCs in blood of patients with MS and the proportion of tumor necrosis factor-α– or CD150-expressing slanDCs. Long-term interferon-β treatment decreases the percentage of blood-circulating slanDCs in contrast to glatiramer acetate or natalizumab. Furthermore, interferon-β inhibits the secretion of proinflammatory cytokines by slanDCs and their capacity to promote proliferation and differentiation of T cells.
Conclusion:
Accumulation of slanDCs in highly inflammatory brain lesions and their presence in CSF indicate that slanDCs may play an important role in the immunopathogenesis of MS. The reduction of blood-circulating slanDCs and the inhibition of their proinflammatory properties by methylprednisolone and interferon-β may contribute to the therapeutic efficiency of these drugs in patients with MS.
Multiple sclerosis (MS) is a frequent chronic inflammatory disease of the CNS that is characterized by damage of myelin sheaths and axonal loss. Various studies revealed that activated CD4+ T helper (Th) cells and CD8+ cytotoxic T lymphocytes (CTLs) essentially contribute to the inflammatory processes underlying MS.1,2 Dendritic cells (DCs) display a unique capacity to induce and expand CD4+ T cells and CD8+ CTLs.3 Recent mouse models demonstrated that DCs essentially contribute to T-cell–mediated inflammation and damage in experimental autoimmune encephalomyelitis.4–10 However, little is known about the role of distinct human DC subsets in MS pathogenesis.
Previously, we described 6-sulfo LacNAc+ (slan) DCs (formerly termed M-DC8+ DCs) as a particular proinflammatory subset of human blood DCs.11–13 We demonstrated that activated slanDCs produce large amounts of various proinflammatory cytokines, display a marked capability to handle immunoglobulin (Ig) G–complexed antigens, and exhibit direct cytotoxic activity.12–16 They also efficiently induce and expand CD4+ T cells and CD8+ CTLs, promote the differentiation of naive CD4+ T lymphocytes into Th1 or Th17/Th1 cells, and activate natural killer cells.11–14,17,18 SlanDCs can be detected in affected tissues of patients with various inflammatory diseases.13,14,19,20
Here, we determined the presence of slanDCs in demyelinated brain lesions and CSF of patients with MS. Furthermore, the impact of drugs approved for MS treatment on the frequency of circulating-blood slanDCs in patients with MS was explored. In addition, the influence of interferon (IFN)-β on various properties was evaluated.
METHODS
Study approval.
All experiments were approved by the institutional review boards of the University Medical Centre Göttingen or the University Hospitals of Ulm or Dresden. Patients or healthy donors gave their written informed consent.
Brain tissues, CSF, and blood from patients or healthy donors.
Brain biopsies of patients with MS were used for immunohistochemical staining. One to 2 MS lesions of different brain regions from 15 patients were analyzed. MS lesions were evaluated based on neuropathologic criteria.21
CSF samples from 9 patients diagnosed with MS according to the revised McDonald criteria,22 9 patients with noninflammatory neurologic disorders (primary headache, musculoskeletal pain, CNS ischemia), 5 patients with viral meningitis, and 5 patients with neuroborreliosis were analyzed for slanDCs.
To assess the impact of immunomodulatory drugs on circulating-blood slanDCs and monocytes from patients with MS, groups of different treatment modalities were selected. Patients diagnosed with relapsing-remitting MS (RRMS) and with stable disease were chosen. Altogether, blood samples were analyzed from healthy donors; untreated patients with RRMS; patients with RRMS treated >18 months with IFN-β (Rebif, Merck Serono Europe Limited, London, UK), glatiramer acetate (GA; Copaxone, Teva Pharmaceuticals, Petah Tikva, Israel), or natalizumab (NA; Tysabri, Biogen-Idec, Weston, MA) (each group comprised 30 patients with MS or healthy donors; 4 patients developed neutralizing anti-IFN-β antibodies during treatment).
We also evaluated the impact of methylprednisolone (MP) on blood slanDCs and monocytes from 11 patients with RRMS with disease relapse. MP was administered IV for 3 days (1 g per day). SlanDCs and monocytes were analyzed before and 1, 2, and 7 days after the first MP infusion.
Immunohistochemical and immunocytochemical staining.
Formalin-fixed and paraffin-embedded brain tissues from patients with MS or ischemic cerebral stroke were cut into 5 µm sections, deparaffinized 3 × 10 minutes in xylene (VWR International, Fontenay-sous-Bois, France), and hydrated to water (B. Braun, Melsungen, Germany) by graded washes of ethanol (Berkel AHK, Ludwigshafen, Germany). Tissue sections were boiled in citrate buffer (Zytomed Systems GmbH, Berlin, Germany) at pH 6 for antigen retrieval. Thereafter, tissue samples were stained with the monoclonal mouse anti-slan antibody DD2 (1:20; Institute of Immunology, Medical Faculty, TU Dresden, Germany) overnight at 4°C. Subsequently, a biotin-labeled anti-mouse IgM antibody (Dako Cytomation, Glostrup, Denmark) was added for 30 minutes. SlanDCs were visualized by the Dako Real detection system (alkaline phosphatase/red; Dako Cytomation). Tissue sections were counterstained with Mayer's hematoxylin (Merck, Darmstadt, Germany).
Immunohistochemical analysis of macrophages and CD3+ T cells was performed as described previously.23,24 Macrophages were identified by staining with the monoclonal mouse anti-KiM1P antibody (1:5,000; Department of Neuropathology, University Medical Centre, Göttingen, Germany) and T cells were identified by using the monoclonal rat anti-human CD3 antibody (1:200; Serotec, Düsseldorf, Germany), which were applied overnight at 4°C. After washing procedure, staining with biotin-labeled anti-mouse IgG antibody (KiM1P) or anti-rat IgG antibody (CD3) was performed for 60 minutes followed by ExtrAvidin-Peroxidase (all Sigma–Aldrich, Steinheim, Germany) for 60 minutes. Antibody binding was detected with the peroxidase substrate 3,3-diaminobenzidine (Sigma–Aldrich). Degree of demyelination was evaluated by luxol fast blue–periodic acid-Schiff staining. Stained sections were analyzed by Olympus BX40 microscope (Olympus, Hamburg, Germany) and evaluated with CellA software. For quantification of cells in tissue sections, positive stained cells were counted at 100× magnification in 10–15 different fields and average density per square millimeter was calculated.
CSF samples from patients with MS and controls were spun down (3,000g for 10 minutes) on slides and air dried for fixation. To detect slanDCs, cytospins were incubated overnight with antibody DD2 at 4°C. After washing procedure, samples were incubated with a biotin-labeled anti-mouse IgM antibody for 30 minutes. SlanDCs were visualized by the Dako Real detection system as described above. Stained cytospins were analyzed by Zeiss Axiophot microscope (Zeiss, Jena, Germany) and evaluated with metaview software. DD2-positive cells were counted at 100× magnification and average density was calculated per 100 leukocytes detected on the slides.
Fluorescence-activated cell sorting.
Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque (Biochrom, Berlin, Germany) density centrifugation. To determine the frequency of slanDCs, PBMCs were incubated with the slan-reactive antibody M-DC8 followed by FITC-conjugated anti-mouse IgM-specific goat F(ab)2 (Beckmann Coulter, Marseille, France) and PerCP-conjugated anti-HLA-DR (BD Biosciences, Heidelberg, Germany), each for 15 minutes at 4°C. FITC-conjugated anti-mouse IgM without primary antibody was used as negative control. Monocytes were characterized by PerCP-conjugated anti-HLA-DR and APC-conjugated anti-CD14 (BD Biosciences). Negative controls included directly labeled or unlabeled isotype-matched irrelevant antibodies (BD Biosciences).
For further characterization of slanDCs and monocytes during relapse treatment, PBMCs were suspended in culture medium consisting of RPMI 1640 (Biochrom), 5% human AB serum (CC pro, Neustadt, Germany), 2 mM l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (Biochrom). PBMCs (2 × 105 cells/well) were plated on round-bottomed 96-well plates. After 6 hours, PBMCs were stimulated with 100 ng/mL lipopolysaccharide (LPS; Sigma–Aldrich) or 10 µM R848 (InvivoGen, Toulouse, France) in the presence of 0.2 µM Monensin (Biomol, Hamburg, Germany) for tumor necrosis factor (TNF)-α staining and harvested 12 hours later. For analysing CD150 expression, cells were incubated with LPS or R848 for 24 hours.
Intracellular molecules were investigated by cell fixation with ice-cold 4% paraformaldehyde (Merck) for 15 minutes and permeabilized with 0.1% saponin (Merck) in phosphate-buffered saline (PBS; Biochrom) plus 1% fetal calf serum (FCS; Biochrom) for 3 minutes at 4°C. Afterward, cells were incubated with a PE-conjugated anti-TNF-α or PE-conjugated isotype-matched irrelevant antibody (BD Biosciences) for 15 minutes. Direct immunofluorescence staining of the cell surface molecule CD150 was performed using PE-conjugated anti-CD150 or PE-conjugated isotype-matched irrelevant antibody (BD Biosciences). Cells were evaluated on a FACS-Calibur cytometer (BD Biosciences).
Immunomagnetic isolation of slanDCs and CD4+ T cells.
Blood samples were drawn from healthy donors. Isolation of slanDCs was performed as described previously.13 The purity of the isolated slanDCs was >90% as determined by flow cytometry. The isolation of CD4+ T cells, CD8+ T cells, and naïve CD45RA+ CD4+ T cells was conducted by depletion from PBMCs utilizing immunomagnetic separation according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). Flow cytometric analysis showed a purity of >90% of the separated T cells.
Cytokine assay.
SlanDCs from healthy donors were cultured for 24 hours in the presence or absence of 103 or 104 U/mL IFN-β. Cells were stimulated with LPS to release cytokines. After another 18 hours supernatants were collected. A commercial ELISA kit (BD Biosciences) was used to measure concentration of cytokines including TNF-α, interleukin (IL)-1β, IL-6, and IL-12p70, following manufacturer's instructions.
T-cell proliferation and programming.
Separated slanDCs were cultured in the absence or presence of 103 or 104 U/mL IFN-β for 6 hours and washed. To perform T-cell proliferation assay, IFN-β–treated slanDCs (1 × 104 cells/well) and allogeneic CD4+ T cells or CD8+ T cells (1 × 105 cells/well) were co-incubated for 4 days. 3H-thymidine (1 µCi, Hartmann Analytic, Braunschweig, Germany) was added for another 18 hours. Then, harvested cells were evaluated for 3H-thymidine incorporation by using a beta counter (PerkinElmer Life Sciences, Rodgau-Jügesheim, Germany).
For evaluation of T-cell programming, IFN-β–treated slanDCs (1 × 104 cells/well) were co-cultured with allogeneic naïve CD45RA+ CD4+ T cells (1 × 105 cells/well) and stimulated with LPS. After 8 days, 10 ng/mL phorbol myristate acetate (Merck) and 1 µg/mL ionomycin (Sigma–Aldrich) were used to restimulate T cells for another 4 hours. To evaluate IFN-γ and IL-4 release, cells were fixed with ice-cold 4% paraformaldehyde for 15 minutes and incubated with 0.1% saponin in PBS (Biochrom) plus 1% FCS for permeabilization for 3 minutes at 4°C. Afterward, cells were incubated with FITC-conjugated anti-IFN-γ and PE-conjugated anti-IL-4 antibody or a PE- or FITC-conjugated isotype-matched irrelevant antibody (all BD Biosciences). After 15 minutes, cells were washed and analyzed by flow cytometry.
Statistical analysis.
Student t test was used to assess the significance of the results. Values of p < 0.05, p < 0.01, and p < 0.001 were considered as significant.
RESULTS
Accumulation of slanDCs in highly inflammatory brain lesions of patients with MS.
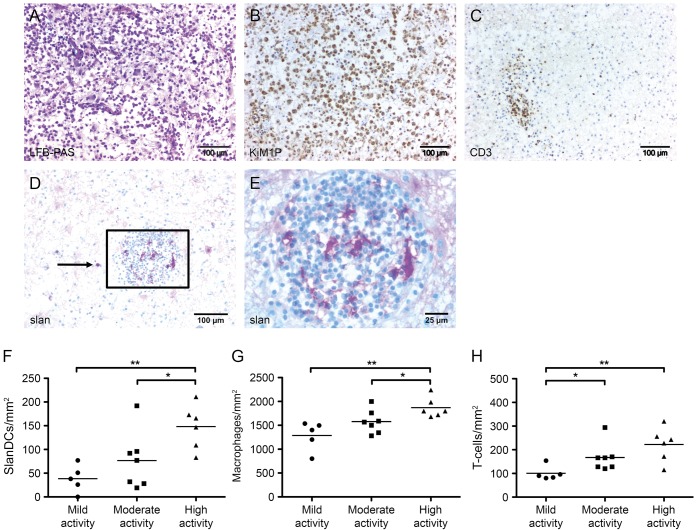
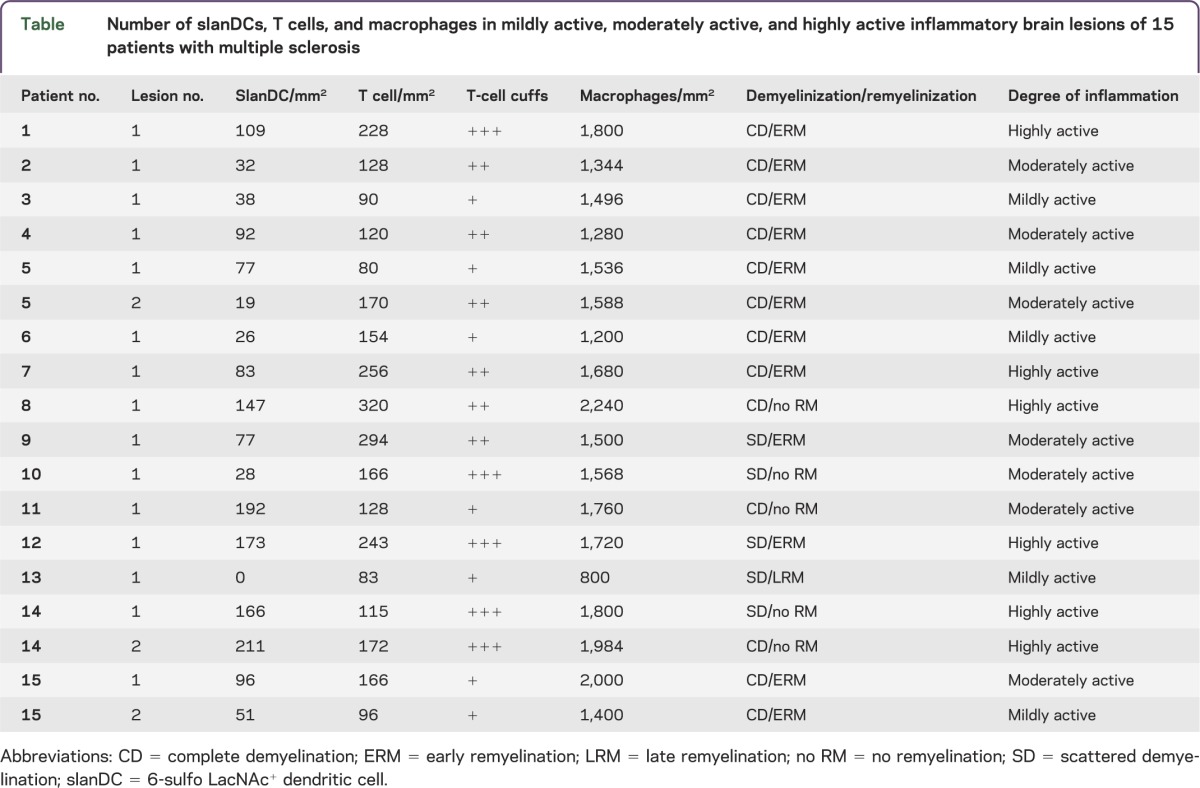
To gain novel insights into the potential role of slanDCs in MS immunopathogenesis, we evaluated the presence of this DC subset in 18 inflammatory demyelinating brain lesions (figure 1A), which were infiltrated by macrophages (figure 1B) and CD3+ T cells (figure 1C). SlanDCs were detected in 17 brain lesions and appeared as irregular-shaped cells with long cytoplasmic processes or round cells in areas with less cell infiltration (figure 1, D and E, table). It is interesting that significantly higher numbers of slanDCs were found in highly active lesions (mean: 155 slanDC/mm2, range: 83–211) compared to lesions with less inflammatory activity (moderate activity—mean: 77 slanDC/mm2, range: 19–192; mild activity—mean: 38 slanDC/mm2, range: 0–77), as shown in figure 1F and the table. Macrophages and CD3+ T cells were also detected at significant frequencies in highly active lesions (figure 1, G and H, table).
Figure 1. Detection of macrophages, T cells, and slanDCs in MS lesions.
In multiple sclerosis (MS) lesions, demyelinated areas were visualized by luxol fast blue–periodic acid-Schiff (LFB-PAS) staining (A). Immunohistochemical stainings were performed to detect macrophages, T cells, and 6-sulfo LacNAc+ dendritic cells (slanDCs) in brain lesions of patients with MS using anti-KiMP1 antibodies (B), anti-CD3 antibodies (C), and anti-slan antibodies (D and E), respectively. All tissue sections were counterstained with Mayer's hematoxylin. Original magnification was ×100. (F–H) The number of slanDCs (F), macrophages (G), and T cells (H) in mildly, moderately, and highly inflammatory brain lesions of patients with MS per mm2 are depicted. Degree of inflammation was defined by the number of perivascular T-cell cuffs (absent [−], low activity: 1 cuff [+], moderate activity: 2 cuffs [++], high activity: 3 or more cuffs [+++]); the number of infiltrating T cells (mild activity: ≤100 cells/mm2, moderate activity: 100–200 cells/mm2, high activity: ≥200 cells/mm2); and the number of infiltrating macrophages (low activity: ≤1,200 cells/mm2, moderate activity: 1,200–1,600 cells/mm2, high activity: ≥1,600 cells/mm2). Horizontal bars show the mean values. Asterisks indicate a statistically significant difference (*p < 0.05, **p < 0.01).
Table.
Number of slanDCs, T cells, and macrophages in mildly active, moderately active, and highly active inflammatory brain lesions of 15 patients with multiple sclerosis
Detection of slanDCs in CSF of patients with MS.
We also determined the presence of slanDCs in CSF samples of 9 patients with MS and 9 control patients with noninflammatory neurologic diseases. SlanDCs were present in 6 out of 9 CSF samples of patients with MS (figure 2, A and B). In contrast, slanDCs were absent in all analyzed control CSF samples (figure 2B). In addition to the accumulation of slanDCs in highly inflammatory brain lesions, these results provide evidence for a contribution of slanDCs to MS immunopathogenesis. For comparison, the presence of slanDCs in CSF samples of patients with other inflammatory disorders was investigated. As depicted in figure 2B, slanDCs were detectable in CSF samples of 4 out of 5 patients with viral meningitis and 3 out of 5 patients with neuroborreliosis.
Figure 2. Detection of slanDCs in CSF of patients with MS.
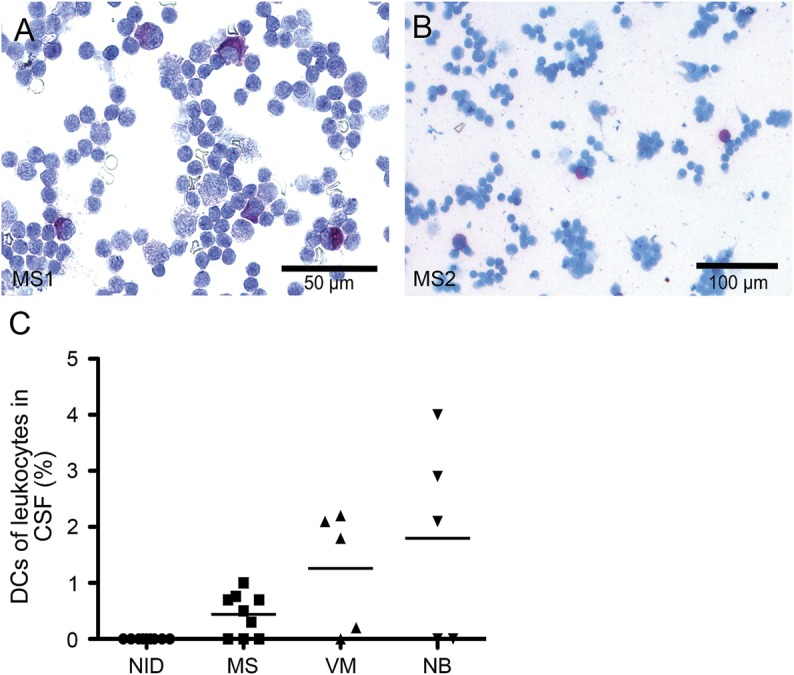
(A, B) Immunocytochemical stainings were performed to determine the presence of 6-sulfo LacNAc+ dendritic cells (slanDCs) in CSF samples of patients with multiple sclerosis (MS), noninflammatory neurologic disorders (NID), viral meningitis (VM), or neuroborreliosis (NB) using anti-slan antibodies. All cytospins were counterstained with Mayer's hematoxylin. Original magnification was ×100. Two representative samples of patients with MS are presented. (C) The percentages of slanDCs in leukocytes derived from CSF samples are depicted. The horizontal bar shows the mean value.
MP decreased the percentage of blood slanDCs in patients with MS.
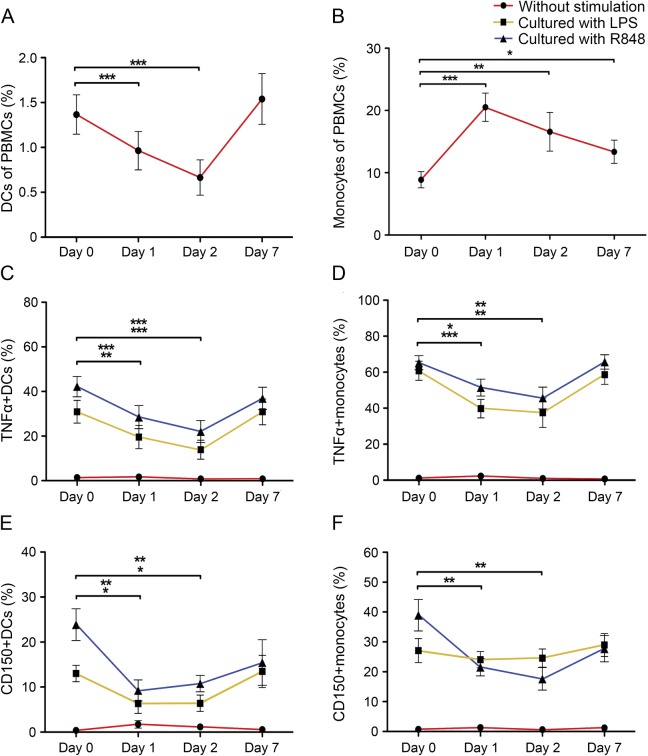
In further experiments, the impact of short-term administration of MP on the percentage of blood slanDCs and monocytes in PBMCs from 11 patients with RRMS with disease relapse was explored. Remarkably, the percentage of slanDCs decreased significantly at day 1 and day 2 of MP treatment (figure 3A). In contrast, the percentage of monocytes significantly increased at these time points (figure 3B).
Figure 3. Impact of short-term MP administration on frequency and phenotype of slanDCs and monocytes in the blood of patients with MS.
The percentages of (A) 6-sulfo LacNAc+ dendritic cells (slanDCs) or (B) monocytes in peripheral blood mononuclear cells (PBMCs) derived from 11 patients with relapsing-remitting multiple sclerosis (RRMS) before and during methylprednisolone (MP) treatment are presented. (C–F) PBMCs derived from the blood of 11 patients with RRMS before and during MP treatment were stimulated with lipopolysaccharide (LPS) or R848. The percentages of tumor necrosis factor (TNF)-α–expressing slanDCs (C) or monocytes (D) as well as CD150-expressing slanDCs (E) or monocytes (F) in PBMCs are demonstrated. Values represent the mean ± SEM of results. The upper row of asterisks indicates a statistically significant difference between R848-activated slanDCs or monocytes on day 1 or day 2 compared to day 0. The lower row of asterisks indicates a statistically significant difference between LPS-activated slanDCs or monocytes on day 1 or day 2 compared to day 0. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001).
Following our recent finding that LPS- or R848-activated slanDCs secrete large amounts of various proinflammatory cytokines,12,19 we investigated the influence of MP on the percentage of TNF-α–expressing slanDCs and monocytes. Therefore, PBMCs from untreated and MP-treated patients with RRMS with acute relapse were stimulated with LPS or R848. Both molecules efficiently increase the percentage of TNF-α–expressing slanDCs and monocytes from untreated patients with RRMS (figure 3, C and D). MP treatment of patients with RRMS significantly reduced the percentage of TNF-α–expressing slanDCs and monocytes after stimulation with LPS or R848 (figure 3, C and D). This effect was particularly pronounced at day 1 and 2 of MP administration.
In addition, the impact of MP on the percentage of slanDCs and monocytes expressing the activation marker CD150 was determined. LPS or R848 efficiently increased the percentage of CD150-expressing slanDCs and monocytes from untreated patients with RRMS (figure 3, E and F). The percentage of CD150-expressing slanDCs and monocytes after stimulation with LPS or R848 was significantly reduced by MP treatment of patients with RRMS (figure 3, E and F).
IFN-β reduced the percentage of blood slanDCs in patients with MS.
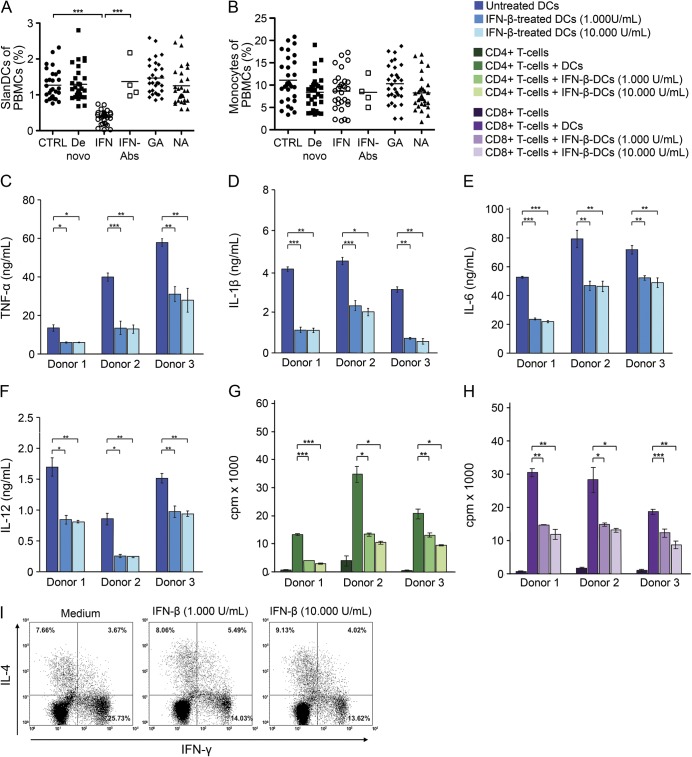
We also explored the impact of long-term treatment of patients with RRMS with IFN-β, GA, or NA on the percentage of slanDCs and monocytes in PBMCs. GA and NA therapy did not influence the percentage of slanDCs and monocytes compared to untreated patients with MS (figure 4, A and B). In contrast to monocytes, IFN-β administration significantly reduced the percentage of slanDCs in PBMCs of all patients with RRMS compared to untreated patients with MS (figure 4, A and B). Notably, the percentage of slanDCs in PBMCs of patients with RRMS who developed neutralizing antibodies during IFN-β treatment was comparable to that of untreated patients with RRMS (figure 4A), indicating that neutralizing anti-IFN-β antibodies can abrogate the effect of IFN-β on slanDCs.
Figure 4. Impact of IFN-β on relevant proinflammatory capabilities of slanDCs.
The percentages of (A) 6-sulfo LacNAc+ dendritic cells (slanDCs) or (B) monocytes in peripheral blood mononuclear cells (PBMCs) derived from blood of 30 patients with relapsing-remitting multiple sclerosis (RRMS) treated with interferon (IFN)-β, glatiramer acetate (GA), or natalizumab (NA); untreated patients with RRMS (de novo); and healthy donors (CTRL) are depicted. In addition, the percentages of (A) slanDCs or (B) monocytes in PBMCs derived from blood of 4 IFN-β-treated patients with RRMS who developed neutralizing anti-IFN-β antibodies (Abs) are shown. Horizontal bars show the mean values. After immunomagnetic cell sorting, slanDCs were stimulated with lipopolysaccharide (LPS) and cultured with or without IFN-β for 24 hours. Supernatant concentrations of (C) tumor necrosis factor (TNF)-α, (D) interleukin (IL)-1β, (E) IL-6, and (F) IL-12p70 were evaluated by ELISA. IFN-β pretreated slanDCs were co-cultured with allogeneic (G) CD4+ or (H) CD8+ T cells. After 4 days 3H-thymidine incorporation was examined to assess T-cell proliferation. Findings of 3 different analyses are presented as mean ± SEM of triplicate determinations. (I) IFN-β-treated slanDCs were co-cultured with allogeneic naive CD45RA+ CD4+ T cells and stimulated with LPS. After 8 days of cell culture, expression of IFN-γ or IL-4 of CD4+ T cells was evaluated by flow cytometry. Three independent analyses presented similar results. Results of one representative donor are presented. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001).
IFN-β impaired cytokine production and slanDC-mediated T-cell proliferation and programming.
In further studies, the impact of IFN-β on important immunostimulatory properties of slanDCs was investigated. IFN-β significantly impaired the capacity of LPS-stimulated slanDCs to produce TNF-α, IL-1β, IL-6, and IL-12p70 (figure 4, C–F). SlanDC-mediated proliferation of CD4+ and CD8+ T cells was also significantly reduced by IFN-β (figure 4, G and H). In addition, the ability of LPS-stimulated slanDCs to promote the differentiation of naïve CD45RA+ CD4+ T lymphocytes into IFN-γ–expressing proinflammatory Th1 cells was markedly inhibited by IFN-β (figure 4I). These results indicate that IFN-β efficiently impairs important immunostimulatory properties of slanDCs.
DISCUSSION
Emerging evidence revealed that human DCs are present in brain lesions and CSF of patients with MS. Thus, the presence of rare DCs expressing the maturation marker CD83 in small numbers of perivascular cuffs in 8 out of 51 tissues from patients with MS has been reported.25 Furthermore, it was demonstrated that mature CD83+ DCs preferentially located in perivascular cuffs of active MS lesions can produce the proinflammatory cytokine IL-23p19.26 DC-SIGN+ DCs expressing the costimulatory molecule CD86 were also detected in perivascular cuffs of MS lesions,27 which can be explained by recent data, indicating that human monocytes migrate across the human blood-brain barrier and differentiate into CD83+ DC-SIGN+ DCs.28
Whereas these studies are based on the detection of general marker molecules for DCs, little is known about the presence of distinct human DC subsets in brain tissues of patients with MS. Previously, human plasmacytoid DCs (pDCs) were reported, which are implicated in the pathogenesis of several inflammatory diseases29 and accumulate in active lesions and inflamed meninges of patients with MS.30 Here, we found that human myeloid slanDCs are detectable in 17 of 18 brain lesions. Remarkably, a significant increase in slanDC number was observed in highly inflammatory brain lesions compared to lesions with less inflammatory activity. Recently, we have documented that slanDCs express the chemokine receptor CXCR4 and that the corresponding ligand CXCL12 promotes their migration.14 Various studies revealed that CXCL12 is expressed in active as well as inactive MS lesions.31–33 However, the expression level of CXCL12 was shown to be higher in active MS lesions than inactive lesions.32 Thus, the elevated CXCL12 expression and/or the presence of additional chemokines such as CXCL1333 in active MS lesions may essentially contribute to the pronounced recruitment of slanDCs in such lesions. SlanDCs were also detectable in the majority of CSF samples. This observation further substantiates previous studies, indicating that numbers of pDCs in CSF of patients with MS are higher than in noninflammatory diseases.34,35 The presence of slanDCs and pDCs in active brain lesions and CSF provide evidence that these human DC subsets may participate in MS immunopathogenesis.
In further studies, the impact of various drugs approved for MS treatment on frequency and properties of slanDCs was explored. Short-term administration of MP significantly decreased the percentage of slanDCs in PBMCs from patients with RRMS. This is in agreement with a previous report demonstrating that MP therapy in patients with MS resulted in a decreased percentage of blood-circulating pDCs and CD11c+ myeloid DCs.36 The MP-mediated reduction of circulating-blood DCs may be caused by inducing apoptosis.37 MP treatment of patients with RRMS also significantly reduced the proportion of TNF-α– or CD150-expressing slanDCs after stimulation compared to slanDCs from untreated patients with RRMS. The inhibitory effects of glucocorticoids have also been reported by other studies, demonstrating that these agents can efficiently impair differentiation, maturation, and functional properties of DCs.38,39
Here, we also described that long-term IFN-β treatment in patients with RRMS significantly decreases the percentage of slanDCs in PBMCs compared to untreated patients with MS, which can be explained by IFN-β–mediated induction of apoptosis.40 Notably, the proportion of slanDCs from patients with RRMS who developed anti-IFN-β antibodies during treatment was comparable to that of untreated patients with RRMS. These findings indicate that neutralizing anti-IFN-β antibodies can abrogate the effect of IFN-β on slanDCs. In addition, we found that IFN-β significantly inhibits the secretion of proinflammatory cytokines by activated slanDCs. The capacity of slanDCs to stimulate CD4+ and CD8+ T-cell proliferation and to promote the polarization of naïve CD45RA+ CD4+ T lymphocytes into proinflammatory Th1 cells was also markedly impaired by IFN-β. These results are in line with previous reports demonstrating that IFN-β impairs the maturation and cytokine release of pDCs and their capacity to stimulate T-cell responses.41,42
Here, we demonstrate that slanDCs accumulate in highly inflammatory brain lesions and are detectable in CSF samples from patients with MS. These novel findings provide evidence that slanDCs may play a role in MS immunopathogenesis. MP and IFN-β significantly decrease the frequency of blood-circulating slanDCs in patients with MS and efficiently impair proinflammatory properties of this DC subset. These effects may contribute to the therapeutic efficiency of these drugs in patients with MS.
ACKNOWLEDGMENT
The authors thank Bärbel Löbel, Karin Günther, and Nick Zimmermann for technical support.
GLOSSARY
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- FCS
fetal calf serum
- GA
glatiramer acetate
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- LPS
lipopolysaccharide
- MP
methylprednisolone
- MS
multiple sclerosis
- NA
natalizumab
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- pDC
plasmacytoid DC
- RRMS
relapsing-remitting MS
- slan
6-sulfo LacNAc+
- Th
T helper
- TNF
tumor necrosis factor
AUTHOR CONTRIBUTIONS
Study concept and design: W. Brück, C. Günther, H. Reichmann, K. Schäkel, M. Schmitz, T. Ziemssen. Acquisition of data: K. Dietze, I. Metz, K. Thomas, R. Wehner. Analysis and interpretation of data: W. Brück, C. Günther, K. Schäkel, K. Thomas. Drafting of the manuscript: M. Schmitz, K. Thomas, T. Ziemssen. Critical revision of the manuscript for important intellectual content: W. Brück, H. Reichmann, K. Schäkel, M. Schmitz, H. Tumani, T. Ziemssen. Statistical analysis: K. Dietze, K. Thomas. Administrative, technical, and material support: W. Brück, C. Günther, K. Schäkel, M. Schmitz, T. Schultheiß, H. Tumani, T. Ziemssen. Study supervision: M. Schmitz, T. Ziemssen.
STUDY FUNDING
This work was supported by grants of the German Research Foundation to Claudia Günther, by grants of the Medical Faculty, Dresden University of Technology, and by an unrestricted research grant of Novartis.
DISCLOSURE
K. Thomas received honorarium from Novartis and Bayer. K. Dietze and R. Wehner report no disclosures. I. Metz received funding from Biogen Idec, Bayer Healthcare, TEVA, Serono, and Novartis; and received research support from German Ministry for Education and Research and Biogen Idec. H. Tumani is on the advisory boards for Biogen Idec, Siemens Health, and Teva Pharma; received funding from Merck-Serono, Teva, Biogen, and Novartis; is a section editor for Neurology, Psychiatry and Brain Research; received research support from Merck-Serono, Teva Pharma, Novartis, Siemens Health, Biogen Idec, Roche Diagnostics, and BMBF; and has done educational presentations for Merck-Serono, Bayer, Teva, and Biogen. T. Schultheiß reports no disclosures. C. Günther received research grants from the Deutsche Forschungsgemeinschaft, University of Dresden. K. Schäkel reports no disclosures. H. Reichmann is on the scientific advisory board for Abbott, Cephalon, Novartis, TEVA, Lundbeck, GSK, Boehringer Ingelheim, Schering/Bayer Health Care, UCB/Schwarz Pharma, Desitin, Merck-Serono, Solvay, and Zambon; has received travel funding/speaker honoraria from Cephalon, Novartis, TEVA, Lundbeck, GSK, Boehringer Ingelheim, Schering/Bayer Health Care, Pfizer, UCB/Schwarz Pharma, Desitin, Solvay, and Zambon; and was employed by Cephalon, Novartis, TEVA, Lundbeck, GSK, Boehringer Ingelheim, Schering/Bayer Health Care, Pfizer, UCB/Schwarz Pharma, Desitin, and Solvay. W. Brück is on the advisory board for Genzyme, Novartis, Biogen Idec Germany, and Teva Pharma; received honoraria from Teva, Sanofi, Genzyme, Novartis, Merck-Serono, Biogen Idec, and Bayer; is on the editorial board for Acta Neuropathologica, Therapeutic Advances in Neurological Disorders, Multiple Sclerosis International, and Neuropathology and Applied Neurobiology; and received research support from German Research Foundation and German Ministry for Science and Education. M. Schmitz reports no disclosures. T. Ziemssen is on the scientific advisory board for Bayer Healthcare, Biogen Idec, Novartis, Merck-Serono, Teva, Genzyme, and Synthon; has received speaker honorarium from Bayer Healthcare, Biogen Idec, Genzyme, MSD, GSK, Novartis, Teva, Sanofi, and Almirall; and has received research support from Bayer Halthcare, Biogen Idec, Genzyme, Novartis, Teva, and Sanofi. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 2009;9:393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mars LT, Saikali P, Liblau RS, Arbour N. Contribution of CD8 T lymphocytes to the immuno-pathogenesis of multiple sclerosis and its animal models. Biochim Biophys Acta 2011;1812:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419–426 [DOI] [PubMed] [Google Scholar]
- 4.Comabella M, Montalban X, Münz C, Lünemann JD. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurol 2010;6:499–507 [DOI] [PubMed] [Google Scholar]
- 5.Zozulya AL, Clarkson BD, Ortler S, Fabry Z, Wiendl H. The role of dendritic cells in CNS autoimmunity. J Mol Med (Berl) 2010;88:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuyts AH, Lee WP, Bashir-Dar R, Berneman ZN, Cools N. Dendritic cells in multiple sclerosis: key players in the immunopathogenesis, key players for new cellular immunotherapies. Mult Scler 2013;19:995–1002 [DOI] [PubMed] [Google Scholar]
- 7.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 2005;11:328–334 [DOI] [PubMed] [Google Scholar]
- 8.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides preferentially polarize CD4+ TH-17 cells in relapsing EAE. Nat Immunol 2007;8:172–180 [DOI] [PubMed] [Google Scholar]
- 9.Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8+ T cells. Nat Immunol 2013;14:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zozulya AL, Ortler S, Lee JE, et al. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci 2009;29:140–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäkel K, Mayer E, Federle C, Schmitz M, Riethmüller G, Rieber EP. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. Eur J Immunol 1998;28:4084–4093 [DOI] [PubMed] [Google Scholar]
- 12.Schäkel K, Kannagi R, Kniep B, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity 2002;17:289–301 [DOI] [PubMed] [Google Scholar]
- 13.Schäkel K, Von Kietzell M, Hänsel A, et al. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity 2006;24:767–777 [DOI] [PubMed] [Google Scholar]
- 14.Hänsel A, Günther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong Th17/Th1 T-cell responses. J Allergy Clin Immunol 2011;127:787–794 [DOI] [PubMed] [Google Scholar]
- 15.Döbel T, Kunze A, Babatz J, et al. FcgRIII equips immature 6-sulfo LacNAc-expressing dendritic cells (slanDCs) with a unique capacity to handle IgG-complexed antigens. Blood 2013;121:3609–3618 [DOI] [PubMed] [Google Scholar]
- 16.Schmitz M, Zhao S, Schäkel K, Bornhäuser M, Ockert D, Rieber EP. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood 2002;100:1502–1504 [PubMed] [Google Scholar]
- 17.Schmitz M, Zhao S, Deuse Y, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol 2005;174:4127–4134 [DOI] [PubMed] [Google Scholar]
- 18.Wehner R, Löbel B, Bornhäuser M, et al. Reciprocal activating interaction between 6-sulfo LacNAc+ dendritic cells and NK cells. Int J Cancer 2009;124:358–366 [DOI] [PubMed] [Google Scholar]
- 19.Hänsel A, Günther C, Baran W, et al. Human 6-sulfo LacNAc (slan) dendritic cells have molecular and functional features of an important pro-inflammatory cell type in lupus erythematosus. J Autoimmun 2013;40:1–8 [DOI] [PubMed] [Google Scholar]
- 20.Günther C, Blau K, Förster U, Viehweg A, Wozel G, Schäkel K. Reduction of inflammatory slan (6-sulfo LacNAc) dendritic cells in psoriatic skin of patients treated with etanercept. Exp Dermatol 2013;22:535–540 [DOI] [PubMed] [Google Scholar]
- 21.Lucchinetti CF, Brück W, Rodriguez M, Lassmann H. Multiple sclerosis: lessons from neuropathology. Semin Neurol 1998;18:337–349 [DOI] [PubMed] [Google Scholar]
- 22.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vass K, Lassmann H, Wekerle H, Wisniewski HM. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol 1986;70:149–160 [DOI] [PubMed] [Google Scholar]
- 24.Lucchinetti CF, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717 [DOI] [PubMed] [Google Scholar]
- 25.Plumb J, Armstrong MA, Duddy M, Scheithauer B, Rodriguez M, Lassmann H. CD83-positive dendritic cells are present in occasional perivascular cuffs in multiple sclerosis lesions. Mult Scler 2003;9:142–147 [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain 2007;130:490–501 [DOI] [PubMed] [Google Scholar]
- 27.Serafini B, Rosicarelli B, Magliozzi R, et al. Dendritic cells in multiple sclerosis: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol 2006;65:124–141 [DOI] [PubMed] [Google Scholar]
- 28.Ifergan I, Kebir H, Bernard M, et al. The blood-brain barrier induces differentiation of migrating monocytes into TH17-polarizing dendritic cells. Brain 2008;131:785–799 [DOI] [PubMed] [Google Scholar]
- 29.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 2008;8:594–606 [DOI] [PubMed] [Google Scholar]
- 30.Lande R, Gafa V, Serafini B, et al. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-b. J Neuropathol Exp Neurol 2008;67:388–401 [DOI] [PubMed] [Google Scholar]
- 31.Ambrosini E, Remoli ME, Giacomini E, et al. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 2005;64:706–715 [DOI] [PubMed] [Google Scholar]
- 32.Calderon TM, Eugenin EA, Lopez L, et al. A role for CXCL12 (SDF-1a) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol 2006;177:27–39 [DOI] [PubMed] [Google Scholar]
- 33.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006;129:200–211 [DOI] [PubMed] [Google Scholar]
- 34.Pashenkov M, Huang YM, Kostulas V, Haglund M, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 2001;124:480–492 [DOI] [PubMed] [Google Scholar]
- 35.Longhini AL, Von Glehn F, Brandao CO, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J Neuroinflammation 2011;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro J, Aristimuno C, Sánchez-Ramón S, et al. Circulating dendritic cell subsets and regulatory T cells at multiple sclerosis relapse: different short-term changes on corticosteroids therapy. J Neuroimmunol 2006;176:153–161 [DOI] [PubMed] [Google Scholar]
- 37.Abe M, Thompson AW. Dexamethasone preferentially suppresses plasmacytoid dendritic cell differentiation and enhances their apoptotic death. Clin Immunol 2006;118:300–306 [DOI] [PubMed] [Google Scholar]
- 38.Piemonti L, Monti P, Allavena P, Leone BE, Caputo A, Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol 1999;162:6473–6481 [PubMed] [Google Scholar]
- 39.Rozkova D, Horvarth R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol 2006;120:260–271 [DOI] [PubMed] [Google Scholar]
- 40.Yen JH, Ganea D. Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood 2009;114:1344–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lòpez C, Comabella M, Al-zayaz H, Tintoré M, Montalban X. Altered maturation of circulating dendritic cells in primary progressive MS patients. J Neuroimmunol 2006;175:183–191 [DOI] [PubMed] [Google Scholar]
- 42.Balashov KE, Aung LL, Vaknin-Dembinsky A, Dhib-Jalbut S, Weiner HL. Interferon-β inhibits toll-like receptor 9 processing in multiple sclerosis. Ann Neurol 2010;68:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]