A sixty-five year old Hispanic male presented to his local clinic in September 2012 with a new-onset fatigue. On physical examination his liver could be palpated 2 cm and his spleen 3 cm below the costal margins. His hemoglobin level was 10 g/dl and his leukocyte count was 12,000 X 106/ml with 5,200 X 106/ml monocytes. A bone marrow aspiration and biopsy were compatible with chronic myelomonocytic leukemia type 1 (CMML-1). Scattered clusters of small PAX5-positive B-cells and an interstitial infiltrate of CD3-positive T-cells were noted and a cytogenetic analysis revealed an 11q23-25 deletion in 2 metaphases. Polymerase chain reaction (PCR) analysis did not detect BCR-ABL1 transcripts or abnormalities in PDGFRA and PDGFRB. The patient was treated with azacitidine 100 mg intravenously daily, for 7 consecutive days once a month. Despite two cycles of therapy, the patient remained transfusion-dependent and his leucocyte count rose to 86,000 X 106/ml with 53,000 X 106/ml monocytes and 32,000 X 106/ml lymphocytes. Nevertheless, a third cycle of azacitidine was administered and the patient was referred to our institution. A repeat bone marrow aspiration detected 20% monoblasts (Figure 1A, right lower corner) expressing CD11c antigen as assessed by immunohistochemistry (Figure 1D) and 24% monocytes. Flow cytometry analysis detected a large population of cells co-expressing CD45 (dim), CD13, CD15 (partial), CD33, CD4 (dim), CD36 (partial), CD38, CD64 partial, CD117, CD123, MPO, and HLA-DR. Together, these findings were consistent acute myelomonocytic leukemia (AMML), likely transformed from CMML. In addition, a large population of mature small lymphocytes (Figure 1A, left upper corner) co-expressing CD5 (Figure 1B) and CD19 (Figure 1C) antigens was noted. Flow cytometry analysis showed that 15% percent of the total events were those of cells co-expressing CD5 (dim), CD19+, CD20 (dim), CD22, CD23, CD43, CD200 (dim), IgG lambda, but not FMC-7, or CD11c antigens, consistent with a concomitant diagnosis of CLL. Unluckily, as more emphasis was given to the former diagnosis, no prognostic analysis, including Fluorescence In Situ Hybridization and immunoglobulin heavy-chain (IgH) gene rearrangement were performed. Polymerase chain reaction (PCR) analysis revealed FLT3 internal tandem duplication (ITD) with an ITD ratio of 0.221 (a D835 point mutation was not detected). Azacitidine was continued at the same dose and schedule and sorafenib 400 mg twice daily was added. After 1 month of treatment, the patient developed a grade 3-4 anemia and neutropenia and therefore the sorafenib dose was reduced to 200 mg twice daily. No toxicities other than hematological toxicity were noted. After 3 cycles of therapy, the patient’s liver and spleen shrunk and were no longer detected below costal margins and he became transfusion-independent. His hemoglobin level was 10 g/dl, his white blood cell count was 5,900 X 106/ml with no blasts and a normal number of monocytes and lymphocytes in the peripheral blood smear, and his platelet count was 60,000 X 106/ml. A bone marrow aspiration and biopsy showed only 11% myelo-monoblasts and 36% monocytes, compatible with CMML. CLL cell aggregates were no longer detected by morphologic analysis (Figure 1E). Continuation of the same treatment regimen was recommended. At 6 months, the patient was transfusion-independent and his physical exam was normal. His hemoglobin level was 10.1 g/dl, platelet count was 115 X 106/ml, and white blood cells were 2,500 X 106/ml with no blasts and a normal number of monocytes and lymphocytes in the peripheral blood smear. However, a bone marrow aspiration and biopsy was compatible with relapsed AMML with 33% myelo-monoblasts and, in addition, relapsed CLL, interstitial pattern, comprising 5-10% of the total bone marrow cellularity (Figure 1F). PCR analysis detected for the first time a FLT3 D835 mutation (ratio 0.274).
Figure 1.
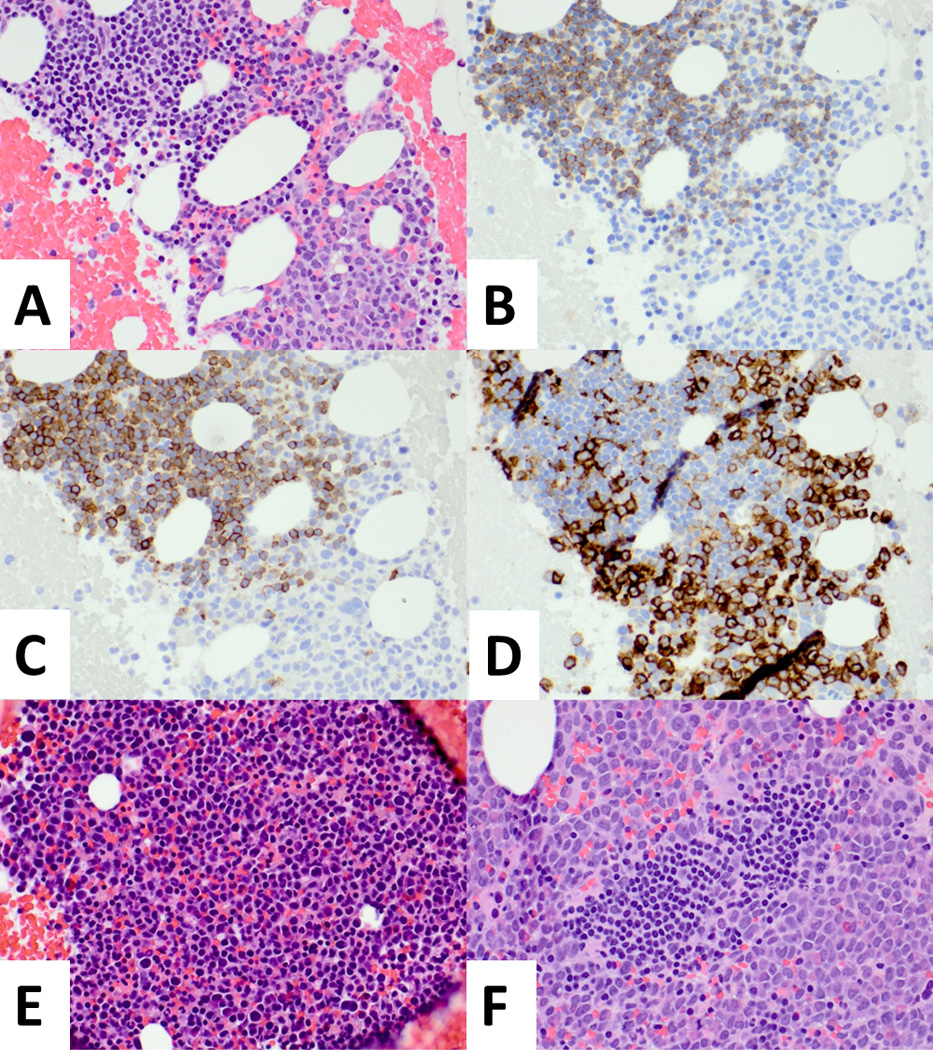
A. Bone marrow clot section shows immature cells with monoblastic morphology (right lower corner) intermixed with mature-appearing lymphocyte (left upper corner), H & E, X40. B and C. The mature-appearing lymphocytes co-express CD5 and CD19, consistent with chronic lymphocytic leukemia (CLL), B-CD19 stain, C-CD5 stain, X40. D. CD11c stain is positive in monoblastic population and negative in CLL population, CD11c stain, x40. E. There is a residual monoblastic population, but CLL population is not observed, H & E, x40. F. CLL population is relapsed (center), demarcated from monoblastic population, H & E, X40.
Several studies established that the microenvironment provides Chronic Lymphocitic Leukemia (CLL) cells with proliferation signals and survival advantage1. An important component of the CLL cell microenvironment consists of monocyte-derived cells. Like solid tumor-associated macrophages2, stromal monocyte-derived nurse-like cells maintain and protect CLL cells. Here we present a patient with myelomonocytic leukemia and CLL in whom the expansion of the CLL clone depended on the presence of neoplastic monocytes. The clinical activity of B-cell receptor signal transduction inhibitors3 raised the possibility that other kinase inhibitors might provide clinical benefits in patients with CLL. Sorafenib is a multi-kinase inhibitor used in various solid tumors. In vitro studies suggested that sorafenib might be active in CLL as it induced apoptosis in primary CLL cells by downregulation of the anti-apoptotic proteins Bcl-2 and Mcl-14, 5. The patient described in this report had both myelomonocytic leukemia and CLL. Whereas the myelomonocytic clone was likely resistant to azacytidine, as progression from CMML to AMML occurred during treatment with azacytidine, it was sensitive to sorafenib, administered because FLT-3 ITD was detected. At the same time the CLL clone, expanded during the myelomonocytic disease progression, diminished when the AMML clone was suppressed. It is unlikely that azacytidine eradicated the CLL clone as it expanded during azacitidine treatment, consistent with clinical data showing that azacitidine had no clinical activity in fludarabine-refractory CLL6. It is also unlikely that sorafenib directly suppressed the CLL clone, as the above mentioned in vitro studies would suggest. In fact, the CLL clone re-surfaced when the AMML clone was no longer responsive to treatment. It is not reasonable that both clones became resistant at the same time. The possibility that both the myelomonocytic and the CLL cells arose from the same hematopoietic clone seems doubtful as FLT-3 mutations have never been detected in CLL. A last possibility is an incidental coexistence and a spontaneous regression of the CLL clone, as previously described7, 8. However, its symmetrical parallelism with the AMML clone points toward this hypothesis. Recent studies confirm the important role monocytes and monocyte-derived cells such as NLCs play in the pathobiology of CLL as an increased numbers of circulating monocytes and monocyte-derived factors were found to be associated with an unfavorable outcome in patients with CLL9, 10. Therefore, it is very likely that in our patient myelomonocytic leukemia cells functioned as NLCs and stimulated the expansion of CLL clone.
References
- 1.Burger JA, Montserrat E. Coming full circle, 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filip AA, Cisel B, Koczkodaj D, Wasik-Szczepanek E, Piersiak T, Dmoszynska A. Circulating microenvironment of CLL: are nurse-like cells related to tumor-associated macrophages? Blood Cells Mol Dis. 2013;50(4):263–270. doi: 10.1016/j.bcmd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fecteau JF, Bharati IS, O'Hayre M, Handel TM, Kipps TJ, Messmer D. Sorafenib-induced apoptosis of chronic lymphocytic leukemia cells is associated with downregulation of RAF and myeloid cell leukemia sequence 1 (Mcl-1) Mol Med. 2012;18:19–28. doi: 10.2119/molmed.2011.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber S, Oelsner M, Decker T, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25(5):838–847. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 6.Malik A, Shoukier M, Garcia-Manero G, et al. Azacitidine in Fludarabine-Refractory Chronic Lymphocytic Leukemia: A Phase II Study. Clin Lymphoma Myeloma Leuk. 2012 doi: 10.1016/j.clml.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawstron AC. Occult B-cell lymphoproliferative disorders. Histopathology. 2011;58(1):81–89. doi: 10.1111/j.1365-2559.2010.03702.x. [DOI] [PubMed] [Google Scholar]
- 8.Robak T, Urbanska-Rys H, Smolewski P, et al. Chronic myelomonocytic leukemia coexisting with B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44(11):2001–2008. doi: 10.1080/1042819031000110946. [DOI] [PubMed] [Google Scholar]
- 9.Mazumdar R, Evans P, Culpin R, Bailey J, Allsup D. The automated monocyte count is independently predictive of overall survival from diagnosis in chronic lymphocytic leukaemia and of survival following first-line chemotherapy. Leuk Res. 2013;37(6):614–618. doi: 10.1016/j.leukres.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Seiffert M, Schulz A, Ohl S, Dohner H, Stilgenbauer S, Lichter P. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116(20):4223–4230. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]


