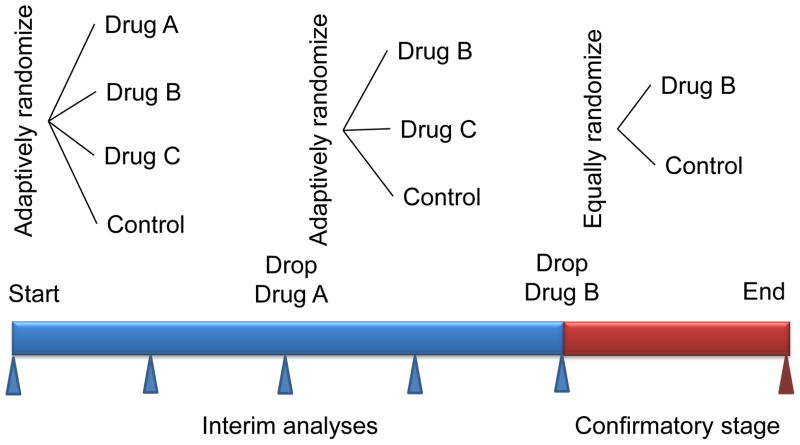
Figure 2. Adaptive trail design for picking the appropriate therapy.
In the example trial, single agent drug B is selected in the phase II part of the trial and continues into phase III. The number of patients in phase II is chosen adaptively. The randomization in the phase II part can also be adaptive, as indicated in the figure. In the phase III part (confirmatory stage) the sample size depends on the results of phase II. Phase III might have interim analyses for stopping accrual early, for either expected success or futility. The drug B versus control element during phase II may (inferentially seamless) or may not (operationally seamless) be counted in the phase III comparison. Controlling the type I error rate in the former case requires simulating the entire trial. (Modified and reprinted with permission Berry, D. A. Adaptive clinical trials in oncology Nat. Rev. Clin. Oncol. 2011; 9: 199–207)