Abstract
Astrocyte-elevated gene-1 (AEG-1/MTDH/LYRIC) is a potent oncogene that regulates key cellular processes underlying disease of the central nervous system (CNS). From its involvement in human immunodeficiency virus (HIV)-1 infection to its role in neurodegenerative disease and malignant brain tumors, AEG-1/MTDH/LYRIC facilitates cellular survival and proliferation through the control of a multitude of molecular signaling cascades. AEG-1/MTDH/LYRIC induction by HIV-1 and TNF highlights its importance in viral infection, and its incorporation into viral vesicles supports its potential role in active viral replication. Overexpression of AEG-1/MTDH/LYRIC in the brains of Huntington’s disease patients suggests its function in neurodegenerative disease, and its association with genetic polymorphisms in large genome-wide association studies of migraine patients suggests a possible role in the pathogenesis of migraine headaches. In the field of cancer, AEG-1/MTDH/LYRIC promotes angiogenesis, migration, invasion, and enhanced tumor metabolism through key oncogenic signaling cascades. In response to external stress cues and cellular mechanisms to inhibit further growth, AEG-1/MTDH/LYRIC activates pathways that bypass cell checkpoints and potentiates signals to enhance survival and tumorigenesis. As an oncogene that promotes aberrant cellular processes within the CNS, AEG-1/MTDH/LYRIC represents an important therapeutic target for the treatment of neurological disease.
Keywords: AEG-1/MTDH/LYRIC, CNS, HIV, Cancer, Glioblastoma
Introduction
The central nervous system (CNS) represents a significant focus for biomedical research due to the relative lack of understanding of the variety of disease pathology that lies within this compartment. A thorough understanding of neurological disease and the discovery and validation of treatment targets have lagged behind many other organ systems within the human body. This relative shortcoming results from the difficulty in studying the CNS, in creating in vitro and in vivo models for CNS disease, and in producing therapeutics that are able to achieve sufficient concentration in targeted areas of the brain. For example, the treatment of neurodegenerative disease remains a challenge due to the lack of validated biomarkers, targets for treatment, and the ability to reverse disease pathogenesis with therapeutic compounds. Similarly, migraines remain an area of particular therapeutic challenge due to a relative lack of understanding of their etiology and molecular pathogenesis. Soon after infection with the human immunodeficiency virus (HIV), the CNS becomes a reservoir of latent virus that remains resistant to systemic treatment using traditional antiretroviral drugs. As a result, a more complete understanding of the way in which HIV enters the CNS and continues to replicate at low levels and the proteins that are responsible for this process could pave the way for enhanced therapeutic efficacy in the future.
In the area of CNS neoplasms, there has been limited progress to improve clinical prognoses. Glioblastoma represents one of the most malignant human cancers, with 5-year survival rates of just 6.9% (Central Brain Tumor Registry of the United States, CBTRUS) (Dolecek et al., 2012) and median survival rates that remain around 12–15 months from the time of diagnosis. Despite optimal treatment with surgical debulking, chemotherapeutic regimens, and radiation protocols, glioblastoma is highly aggressive and typically recurs following treatment. These tumors exhibit a dramatic propensity for mutation, chemo- and radioresistance, and microscopic spread throughout the CNS that provides a difficult substrate for therapeutic intervention. Therefore, this tumor entity represents a particularly attractive target for research in order to achieve progress in overall patient survival.
Astrocyte-elevated gene-1 (AEG-1/MTDH/LYRIC) is an HIV- and tumor necrosis factor (TNF)-inducible oncogene that is upregulated in all human cancers thus far profiled. Initially characterized as a responsive gene to HIV infection, the role of this gene has greatly expanded in the realm of CNS disease and has been shown to regulate neurodegenerative disease, migraines, glutamate signaling, and tumorigenesis. Through a variety of cellular signaling pathways, AEG-1/MTDH/LYRIC promotes cellular survival, the oncogenic state, and coordinates a host of cellular processes that encourage angiogenesis, migration, invasion, and metastasis. Also known as lysine-rich CEACAM-1-associated protein (LYRIC) in rats (Sutherland, Lam, Briers, Lamond, & Bickmore, 2004) and metadherin (MTDH) in humans (Brown & Ruoslahti, 2004), AEG-1/MTDH/LYRIC expression is ubiquitous in vertebrates and may contribute to normal cellular processes that govern growth. However, little is known about the function of AEG-1/MTDH/LYRIC under normal physiological circumstances in nontransformed cells within the CNS.
As a potent regulator of key cellular pathways mediating glioma proliferation, angiogenesis, and chemoresistance, AEG-1/MTDH/LYRIC serves a primary role in well-characterized signaling cascades known to promote tumorigenesis. AEG-1/MTDH/LYRIC enhances the aggressive phenotype in glioblastoma and facilitates its expansion within the skull case through the dissolution of extracellular matrix and death of surrounding cells. By hijacking cellular machinery that interferes with normal checkpoint regulation, AEG-1/MTDH/LYRIC promotes dysregulated tumor growth and associates with poor prognosis in glioma. With evidence suggesting its use as a biomarker in cancer (Chen et al., 2012) and its utility in therapeutic cancer vaccine intervention (Qian et al., 2011), further research into this gene at the center of many signaling cascades may shed light on molecular mechanisms of neurological disease and may provide improved diagnostic and therapeutic tools for patients in the future.
The Role of AEG-1/MTDH/LYRIC in CNS Disease Pathogenesis
The association of AEG-1/MTDH/LYRIC with HIV-1-associated neurocognitive disorder through glutamate excitotoxicity and reactive gliosis
The HIV-1 pandemic continues to represent a significant public health burden and remains a therapeutic challenge. Soon after HIV-1 infection, virus likely enters the CNS through the activation of monocytes in bone marrow and subsequent circulation in the blood. This reservoir of HIV-1 within macrophages is resistant to systemic antiviral treatment and is potentially responsible for the maintenance of low levels of viral replication during treatment. In addition, HIV-1 infection in the CNS eventually leads to the development of HIV-associated neurocognitive disorder and HIV-associated dementia (HAD), which carry significant morbidity for affected patients (Ghafouri et al., 2006 and McArthur et al., 2010). The ability to target this reservoir of viral replication and develop therapeutic options to treat neurological sequelae of HIV-1 infection may prove useful in eliminating latent HIV-1 and low-level replication and improving quality of life in affected patients.
AEG-1/MTDH/LYRIC was initially cloned through a rapid subtraction hybridization approach in primary human fetal astrocytes (PHFA) infected by HIV-1 (Kang et al., 2005). PHFA exhibit an approximately threefold increase in AEG-1/MTDH/LYRIC RNA levels following infection by HIV-1 or treatment with TNF-α (Su et al., 2003), indicating that AEG-1/MTDH/LYRIC may promote HIV-1 infection and its pathogenesis. AEG-1/MTDH/LYRIC/LYRIC physically interacts with HIV-1 Gag and is incorporated into viral particles and cleaved by HIV-1 PR (Engeland et al., 2011). Though the role of AEG-1/MTDH/LYRIC in this context is unknown, it is possible that AEG-1/MTDH/LYRIC thus regulates virion assembly, maturation, or release (Fig. 1).
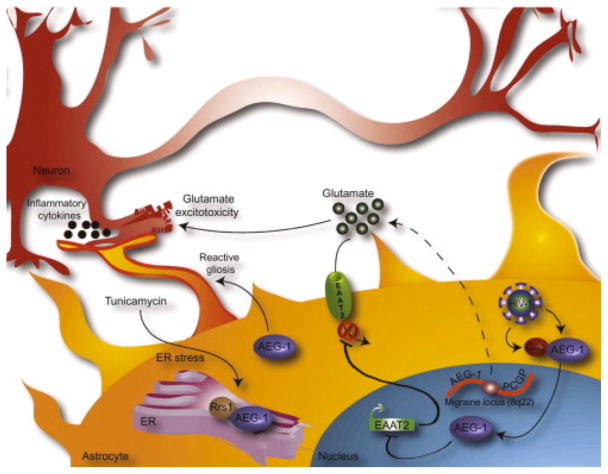
Figure 1. AEG-1/MTDH/LYRIC contributes to the pathogenesis of neurodegenerative disease, glutamate excitotoxicity, reactive gliosis, and migraine headaches.
AEG-1/MTDH/LYRIC is induced by HIV-1 in primary human fetal astrocytes (PHFA) and interacts with HIV-1 Gag protein, potentially promoting HIV-1 virion assembly, maturation, and release. In the nucleus, AEG-1/MTDH/LYRIC also downregulates the EAAT2 promoter, resulting in decreased transporter expression, reduced glutamate uptake from the extracellular space, and a glutamate excitotoxic environment that is toxic to neighboring neurons. AEG-1/MTDH/LYRIC, as well as Rrs1, a protein involved in ribosomal assembly, is induced by the ER stress compound, tunicamycin, and colocalize in the ER, where these proteins may promote tumorigenic functions. In addition, AEG-1/MTDH/LYRIC promotes reactive gliosis following injury and is involved in astrocyte activation and subsequent neurodegeneration. In the area of migraine, a susceptibility locus on 8q21-22 between the genes encoding AEG-1/MTDH/LYRIC and PGCP, a glutamate regulatory molecule, may implicate glutamate excitotoxicity in the pathogenesis of migraine. AEG-1/MTDH/LYRIC, astrocyte-elevated gene-1; EAAT2, excitatory amino acid transporter 2; Rrs1, regulatory of ribosomal synthesis 1, PGCP, plasma glutamate carboxypeptidase.
There is compelling evidence that AEG-1/MTDH/LYRIC generally encourages virus entry into the CNS through the promotion of vascular permeability. Through molecular mimicry, AEG-1/MTDH/LYRIC acts as the target of the dengue virus (DENV) and enhances vascular permeability, potentially allowing for the spread of virus (Liu, Chiu, Chen, & Wu, 2011). Neurological manifestations, including viral encephalitis, are present in 1–5% of patients with symptomatic systemic DENV, and therefore, AEG-1/MTDH/LYRIC may also promote DENV entry or its spread into the CNS. Though AEG-1/MTDH/LYRIC in this case represents a permissive proponent for vascular dispersion, it is conceivable that AEG-1/MTDH/LYRIC may share sequence homology with other viral antigens to enhance virus entry, survival, and spread after infection.
The death of neuronal cells is a known consequence of HIV infection and leads to the development of HAD. One clear mechanism for this neuronal destruction is the accumulation of toxic levels of glutamate, a process termed glutamate excitotoxicity, that is associated with a number of neurological diseases, such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, Parkinson’s disease, and epilepsy. Glutamate is an essential amino acid for cellular growth and is required for a multitude of normal cellular processes, such as neurotransmission within the CNS. However, dysregulated control of extracellular glutamate concentrations can lead to an excitotoxic environment that is detrimental to surrounding cells. In the brain, the primary mechanism by which glutamate mediates its excitotoxic effects is through the expression of the excitatory amino acid transporter-2 (EAAT2), the principal transmembrane ion channel that regulates the concentration of glutamate through extracellular uptake. AEG-1/MTDH/LYRIC has been shown to downregulate the promoter of the EAAT2, rendering these cells more susceptible to glutamate excitotoxicity (Kang et al., 2005). In addition, this regulation appears to be phosphatidyl inositol 3-kinase (PI3K)-dependent, as PTEN overexpression abrogates this promoter silencing. As EAAT2 promoter activity is dependent on epidermal growth factor (EGF) and cAMP, it is possible that AEG-1/MTDH/LYRIC works in a similar fashion to modify glutamate transport.
In addition to glutamate excitotoxicity, a critical mediator of neuronal injury in HIV-1 infection is the process of reactive gliosis, whereby resting astrocytes are activated and produce inflammatory cytokines that damage surrounding normal brain parenchyma (Maragakis & Rothstein, 2006). This process underlies cell damage pathways in many neurodegenerative diseases and is thought to be responsible for some of the CNS complications of HIV-1 infection. AEG-1/MTDH/LYRIC promotes reactive gliosis in vivo and is responsible for astrocyte migration following injury ( Vartak-Sharma & Ghorpade, 2012). In addition, the subcellular localization of AEG-1/MTDH/LYRIC is altered following brain injury, with a significant increase in cytoplasmic AEG-1/MTDH/LYRIC staining and no change in overall expression levels. However, during wound healing, AEG-1/MTDH/LYRIC colocalizes with the nucleolar marker, fibrillarin, indicating that AEG-1/MTDH/LYRIC translocates to the nucleolus, where it may cooperate in transcriptional transactivation of target genes necessary for proliferation and migration. Given the importance of restricted HIV-1 infection of astrocytes in the generation of HIV-1-associated neuropathies and dementia ( Sabri, Titanji, De Milito, & Chiodi, 2003), this represents an alternative mechanism by which AEG-1/MTDH/LYRIC promotes CNS pathogenesis of HIV-1 infection.
The role of AEG-1/MTDH/LYRIC in neurodegenerative disease and migraine
Neurodegenerative diseases compose a major subset of neurological disease and are associated with significant morbidity without any currently available disease-modifying therapeutic options. Huntington’s disease (HD) is an inherited autosomal-dominant neurodegenerative disorder caused by trinucleotide repeat expansion in the HD gene, huntingtin, and characterized clinically by progressive neurological deterioration and then death in the setting of chorea and psychiatric perturbation. In a model of HD using immortalized striatal neuronal cells, LYRIC was found to colocalize with the regulator of ribosome synthesis (Rrs1), a protein important for the maturation, nuclear export, and assembly of ribosomes, in the nucleolus and endoplasmic reticulum (ER) membrane. Moreover, in response to ER stress in vitro, both genes are induced ( Carnemolla et al., 2009). Immunohistochemical analysis of HD brains demonstrates that AEG-1/MTDH/LYRIC is upregulated 1.7-fold over control brains, suggesting that its overexpression may be associated with the pathogenesis of HD. It was hypothesized that given its localization to both ER and nucleolar compartments and the proximity of ER and nucleolar membranes ( Fricker, Hollinshead, White, & Vaux, 1997), LYRIC may act as a sensor of ER stress in this context and transmit these signals to the nucleolus. Though AEG-1/MTDH/LYRIC has not been studied in the context of other neurodegenerative diseases, the fact that many of these diseases, such as Alzheimer’s disease, Parkinson’s disease, and ALS, are marked by ER stress ( Roussel et al., 2013), AEG-1/MTDH/LYRIC may serve a pathogenic function in a host of chronic progressive CNS diseases.
Like neurodegenerative disease, the pathogenesis of migraine headaches is poorly understood, and few effective therapies are available. As a result, genome-wide association studies have been performed to better investigate the genetic predisposition to migraine and stratify patients based on heritability markers. In a recent genome-wide association study (GWAS) study, AEG-1/MTDH/LYRIC was shown to hold a potential role in migraine pathogenesis, with a susceptibility allele for migraine being identified in the region of AEG-1/MTDH/LYRIC on chromosome 8q22 (Anttila et al., 2010). This study also showed that AEG-1/MTDH/LYRIC transcript levels correlated with the rs1835740 genotype and that this genotype may be a cis regulator of AEG-1/MTDH/LYRIC. The migraine susceptibility allele identified in this study is flanked by AEG-1/MTDH/LYRIC and the glutamate regulatory gene, plasma glutamate carboxypeptidase (PGCP), which increases glutamate concentrations in the extracellular space through the cleavage of N-acetyl-l-aspartyl-l-glutamate into N-acetyl aspartate (NAA) and glutamate. Therefore, it is possible that glutamate homeostasis is critical for migraine pathogenesis and that AEG-1/MTDH/LYRIC may regulate these pathways through its downregulation of the glutamate transporter, EAAT2 ( Kang et al., 2005 and Noch and Khalili, 2009). In another GWAS meta-analysis by the Dutch Icelandic migraine genetics consortium, the first population-based GWAS for common migraine, gene-based analysis also identified a possible association of MTDH with migraine ( Ligthart et al., 2011). Like the prior study, the common SNPs were located between MTDH and PGCP on 8q21, further supporting dysregulated glutamate signaling in the pathogenesis of migraine. As AEG-1/MTDH/LYRIC regulates glutamate uptake in normal brain, this strengthens the argument that a relative glutamate excitotoxic environment, either through increased release or reduced uptake, stimulates the occurrence of migraine attacks.
Structure and Localization of Astrocyte-Elevated Gene-1 in the CNS
The full-length AEG-1/MTDH/LYRIC cDNA consists of 3611 bp, excluding the poly-A tail (Kang et al., 2005). The open reading frame from 220 to 1968 nts encodes a putative 582-amino acid protein with a calculated molecular mass of 64 kDa with a pI of 9.33. The similar homologue identified in mouse breast cancer cells, named metadherin (MTDH) (metastasis adhesion protein), contains 579 amino acids ( Brown & Ruoslahti, 2004). Genomic blast search demonstrated that the AEG-1/MTDH/LYRIC gene consists of 12 exons/11 introns and is located at 8q22 where cytogenetic analysis of human gliomas indicated recurrent amplifications. Protein motif analysis, such as simple modular architecture research tool (SMART), predicted a single-transmembrane domain at the N-terminus of the protein (amino acids 50–70) that includes putative dileucine repeats that are thought to be important for protein trafficking ( Kirchhausen, 2000) and was supported by three independent transmembrane protein prediction methods (PSORT II, TMPred, and Hidden Markov Model for TOpology Prediction, HMMTOP). However, PSORT II and TMpred predicted AEG-1/MTDH/LYRIC as a type Ib protein (C-terminal inside) (like 3D3/Lyric), whereas TMHMM and TopPred 2 predicted a type II protein (C-terminal outside), which was verified with metadherin protein ( Brown & Ruoslahti, 2004). Analysis of the amino acid sequence of AEG-1/MTDH/LYRIC further revealed the presence of putative, either monopartite or bipartite, nuclear localization signals (NLS) between amino acids 79–91, 432–451, and 561–580, suggesting import into the nucleus. The existence of a cleavable signal peptide was not evident based on the motif analyses. AEG-1/MTDH/LYRIC is a highly basic protein with many lysine (12.3%) and serine (11.6%) residues, indicating that posttranslational modification of AEG-1/MTDH/LYRIC may be critical for its nuclear import.
Initial characterization of AEG-1/MTDH/LYRIC demonstrated its expression in the perinuclear region and in ER-like structures (Kang et al., 2005). As the ER membranes are contiguous with the nuclear envelope, this expression pattern favors type Ib membrane morphology. In addition to its localization in these areas, AEG-1/MTDH/LYRIC is also located in the nucleolus (Sutherland et al., 2004) and at tight junctions (Britt et al., 2004), possibly due to tight junction assembly mediated by protein kinase C (Andreeva et al., 2001 and Sakakibara et al., 1997). However, despite its three NLS, deletion or mutation of these signals does not prevent the functional activity of AEG-1/MTDH/LYRIC, indicating that there are other molecules critical for AEG-1/MTDH/LYRIC trafficking to the nucleus, such as TNF, that induces its nuclear translocation. Interestingly, fully nuclear AEG-1/MTDH/LYRIC is rarely seen possibly because of the leucine-rich putative nuclear export signal from amino acids 61 to 68. Moreover, predominantly monoubiquitination restricts expression of AEG-1/MTDH/LYRIC to the cytoplasmic compartment after posttranslational modification (Thirkettle, Girling, et al., 2009), an expression pattern associated with poor prognosis in cancer (Thirkettle, Girling, et al., 2009). LYRIC is phosphorylated at Ser297 with 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment ( Schulz et al., 2013), and AEG-1/MTDH/LYRIC is phosphorylated at Thr143 ( Dephoure et al., 2008), Ser298 ( Villen, Beausoleil, Gerber, & Gygi, 2007), Ser84, Ser415, Ser426, Ser308 ( Olsen et al., 2006), and Ser496 ( Olsen et al., 2010), which may govern several of its downstream interaction with oncogenic cascades.
Expression of AEG-1/MTDH/LYRIC is evident in many normal and transformed cell types within the CNS. AEG-1/MTDH/LYRIC is expressed in astrocytes at low levels (Kang et al., 2005) and is also present in transformed striatal neurons as well (Carnemolla et al., 2009). Positive expression of AEG-1/MTDH/LYRIC has been demonstrated in normal blood vessels within the CNS (unpublished results) and in human umbilical vein endothelial cells (Liu et al., 2011). In addition, AEG-1/MTDH/LYRIC is developmentally regulated, with expression being noted in the mid-to-hindbrain, frontonasal processes, and pharyngeal arches early in development (Jeon et al., 2010). Subsequently, AEG-1/MTDH/LYRIC is expressed in the brain and olfactory systems, which suggests a potential role in neurogenesis and early brain modeling.
The Role of AEG-1/MTDH/LYRIC in Brain Tumor Pathogenesis
The molecular interaction of AEG-1/MTDH/LYRIC with tumorigenic cell signaling cascades
Like other systemic malignancies, tumors within the CNS are marked by genetic and acquired mutations that support dysregulated cellular growth. Many of these mutations, such as those involving p53, PTEN, and isocitrate dehydrogenase 1 (IDH1), have been examined extensively in glioblastoma (Purow & Schiff, 2009). In addition, studies of overactive oncogenic signaling pathways, such as PI3K, EGFR, and MAPK, and defects in DNA repair machinery have led to the development of novel therapeutics that have entered clinical trials (Johnson & Chang, 2012). Aside from temozolomide, which has demonstrated clear efficacy in phase III clinical trial for the treatment of newly diagnosed glioblastoma (Stupp et al., 2005), no other chemotherapeutic agent alone has prolonged overall survival in patients with this aggressive neoplasm. Improved therapeutic efficacy requires targeting of related yet synergistic pathways that promote glioblastoma, such as angiogenesis and tumor metabolism. Therefore, it is probable that as genomic and metabolic analyses improve our understanding of pathways that support glioblastoma, future efforts will focus on combination therapy to control aberrant tumor signaling and may achieve greater success.
As a TNF-inducible gene with elevated expression in astrocytes transformed by SV40 T-antigen, telomerase (hTERT), and T24 Ha-Ras ( Kang et al., 2005 and Rich et al., 2001), AEG-1/MTDH/LYRIC was originally thought to play a significant role in the process of tumorigenesis. Since that time, AEG-1/MTDH/LYRIC has been shown to be upregulated in a variety of CNS malignancies, including neuroblastoma ( Lee et al., 2009), oligodendroglioma ( Xia et al., 2010), and glioblastoma ( Emdad et al., 2010 and Liu et al., 2010) and correlates with patient prognosis as well ( Liu, Liu, Han, Zhang, & Sun, 2012). In some malignancies, such as ovarian and breast cancer, certain AEG-1/MTDH/LYRIC alleles are associated with increased risk of cancer, though protein expression levels do not differ between allele types ( Yuan et al., 2012). The initial characterization of AEG-1/MTDH/LYRIC showed that this protein promotes anchorage-independent growth ( Kang et al., 2005). Subsequently, AEG-1/MTDH/LYRIC was determined to act as an oncogene and promotes tumor formation when overexpressed in fibroblast cells alone ( Emdad et al., 2009).
On a molecular level, AEG-1/MTDH/LYRIC interacts with a variety of cell signaling pathways that promote tumorigenesis and interrupts normal checkpoints involved in the control of cellular growth (Fig. 2). Within the nuclear factor kappa B (NFκB) pathway, AEG-1/MTDH/LYRIC activates binding of the two components of NFκB, p50 and p65, to NFκB consensus sequences in the promoters of target genes. As a result, AEG-1/MTDH/LYRIC upregulates several downstream genes, such as intercellular adhesion molecule (ICAM)-3, ICAM-2, selectin E, selectin P ligand, selectin L, toll-like receptor (TLR)-4, TLR-5, FOS, JUN, and IL-8 (Emdad et al., 2006), all of which have known functions in cancer. It appears that AEG-1/MTDH/LYRIC promotes NFκB activation by inducing degradation of the NFκB repressor, IκB. Additionally, AEG-1/MTDH/LYRIC promotes nuclear translocation of NFκB and binds to p65 through amino acids 101–205 (Sarkar et al., 2008). The mechanism by which AEG-1/MTDH/LYRIC promotes NFκB-mediated oncogenic activity involves cooperative binding with cyclic AMP-responsive element-binding protein (CREB)-binding protein (CBP), a known activator of NFκB signaling. In this context, AEG-1/MTDH/LYRIC may act as a bridging molecule between CBP and the NFκB promoter to support transactivation of downstream genes.
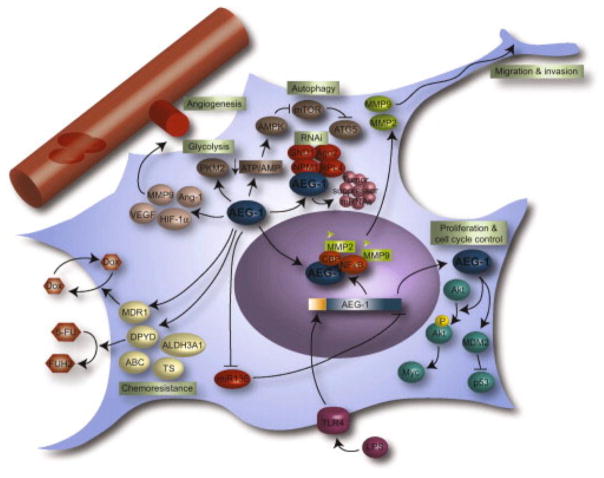
Figure 2. AEG-1/MTDH/LYRIC promotes proliferation, angiogenesis, migration and invasion, tumor-associated cellular metabolism, and chemoresistance in glioblastoma.
AEG-1/MTDH/LYRIC promotes angiogenesis through the induction of MMP9, Ang-1, HIF-1α, and VEGF. The final rate-limiting enzyme in glycolysis, PKM2, is induced by AEG-1/MTDH/LYRIC, which promotes anaerobic glycolysis in glioblastoma. AEG-1/MTDH/LYRIC accelerates ATP consumption and reduces the ATP/AMP ratio, thereby activating AMPK and promoting autophagy through mTOR inhibition and ATG5 activation. Migration and invasion are governed by NFκB-dependent AEG-1/MTDH/LYRIC activation of the MMP2 and MMP9 promoters, a process which is supported by TLR4 activity. In the cytoplasm, AEG-1/MTDH/LYRIC associates with several members of the RNA-induced silencing complex (RISC), including Ago 2, SND1, RPL4, and NPM1 and promotes sequestration of tumor suppressor mRNAs into stress granules. AEG-1/MTDH/LYRIC supports the chemoresistant phenotype through activation of the ABC transporter superfamily, thymidylate synthase, and ALDH3A1. In addition, AEG-1/MTDH/LYRIC promotes doxorubicin efflux through activation of MDR1 and 5-FU metabolism through DPYD activation. AEG-1/MTDH/LYRIC, astrocyte-elevated gene-1; ATP, adenosine trisphosphate; AMPK, 5′-adenosine monophosphate (AMP)-activated protein kinase; mTOR, mammalian target of rapamycin; ATG5, autophagy protein 5; NFκB, nuclear factor kappa B; MMP2, matrix metalloproteinase 2; TLR4, toll-like receptor 4; SND1, staphylococcal nuclease domain-containing 1; RPL4, ribosomal protein L4; NPM1, nucleophosmin 1; TS, thymidylate synthase; ALDH3A1, aldehyde dehydrogenase 3A1; MDR1, multidrug resistance 1; 5-FU, 5-fluorouracil; DPYD, dihydropyridine dehydrogenase.
The PI3K/Akt pathway and a variety of antiapoptotic pathways are critical to initiate and maintain the tumorigenic phenotype in malignant brain tumors (Lino & Merlo, 2011), and AEG-1/MTDH/LYRIC serves an important positive regulatory role in these pathways. AEG-1/MTDH/LYRIC overexpression induces phosphorylation of Akt (Lee et al., 2008), a signaling event that is required for AEG-1/MTDH/LYRIC-mediated tumor induction. Further, AEG-1/MTDH/LYRIC induces phosphorylation and inactivation of glycogen synthase kinase 3β (GSK3β) and concomitant inhibition of p21/mda-6 and the proapoptotic protein, bad. Similarly, expression levels of p53 are reduced in AEG-1/MTDH/LYRIC-overexpressing PHFA along with phosphorylation and activation of the negative regulator of p53, murine double minute 2. AEG-1/MTDH/LYRIC negatively regulates the proapoptotic tumor suppressor, forkhead box class O (FOXO) 3a transcription factor in prostate cancer (Kikuno et al., 2007), which is itself phosphorylated and inhibited by Akt (Brunet et al., 1999), and may thus prevent transcriptional activation of various oncogenic pathways. The Akt target, c-myc, is also induced by AEG-1/MTDH/LYRIC in PHFA (Lee, Su, Emdad, Sarkar, & Fisher, 2006), and AEG-1/MTDH/LYRIC induces n-myc expression in neuroblastoma (Lee et al., 2009). Functionally, AEG-1/MTDH/LYRIC prevents serum starvation-induced apoptosis through a PI3K-dependent mechanism in PHFA. This pathway is dependent on Akt but involves phosphatase and tensin homolog (PTEN), implicating a process downstream of PI3K. In the related extracellular signal-regulated kinase (ERK) pathway, AEG-1/MTDH/LYRIC induces phosphorylation of ERK42/44, p38 mitogen-activated protein kinase (MAPK), and Akt in hepatocellular carcinoma cells, pathways that are critical for AEG-1/MTDH/LYRIC-mediated invasion and anchorage-independent growth (Yoo, Emdad, et al., 2009). Further, AEG-1/MTDH/LYRIC binds to the transcriptional repressor involved in cell cycle control, promyelocytic leukemia zinc finger, and suppresses its transcriptional repression of target genes (Thirkettle, Mills, Whitaker, & Neal, 2009), thereby preventing apoptosis (Bernardo, Yelo, Gimeno, Campillo, & Parrado, 2007). Together, these findings implicate AEG-1/MTDH/LYRIC as a prosurvival and antiapoptotic protein that coordinates with PI3K/Akt signaling to promote tumorigenesis.
Molecules within the Wnt signaling pathway, which regulate patterning and development of the CNS and are also important for certain oncogenic signaling cascades, are modulated by AEG-1/MTDH/LYRIC as well. Lymphoid enhancer-binding factor 1 (LEF1), the transcription factor activated by Wnt signaling, is upregulated, whereas the negative regulators of the Wnt pathway, C-terminal-binding protein 2 and adenomatous polyposis coli, are significantly downregulated by AEG-1/MTDH/LYRIC. Within the Wnt pathway, AEG-1/MTDH/LYRIC enhances phosphorylation of GSK3β and causes nuclear translocation of β-catenin, inducing transcription of the LEF1 promoter (Jian-bo et al., 2011 and Yoo et al., 2009). In hepatocellular carcinoma, LEF1 inhibition abrogates AEG-1/MTDH/LYRIC-induced invasion, highlighting the importance of the Wnt pathway in mediating the tumorigenic effects of AEG-1/MTDH/LYRIC. Given that the Wnt pathway is also dysregulated in glioblastoma (Kaur et al., 2013), it is likely that similar signaling cascades mediate the activity of AEG-1/MTDH/LYRIC in CNS neoplasia.
On a transcriptional level, AEG-1/MTDH/LYRIC contains several E-box elements within its promoter that are targets for oncogenic proteins. The AEG-1/MTDH/LYRIC promoter has positive (− 459/− 302) and negative (− 738/− 460) regulatory regions, with Sp1, E-box element, CREB, and Ets-2 necessary for basal promoter activity (Lee et al., 2006). Two of the E-box elements appear to be necessary for basal and Ras-induced promoter activation in immortalized PHFA, and the negative regulatory region contains putative RAR-α and YY1 binding sites that may repress transcriptional activity. Similarly, oncogenic signaling through the Ha-ras pathway requires the participation of AEG-1/MTDH/LYRIC through c-myc binding to the AEG-1/MTDH/LYRIC promoter ( Lee et al., 2006). Therefore, as a c-myc-dependent target of oncogenic Ras, AEG-1/MTDH/LYRIC may facilitate the coordinated activity of these oncogenic pathways in tumorigenesis. Moreover, the transcriptional regulatory processes that induce AEG-1/MTDH/LYRIC may be suitable substrates for therapeutic investigation.
The role of AEG-1/MTDH/LYRIC in cell cycle dysregulation in glioblastoma
The dysregulation of cell cycle progression is critical to the foundation of cancer, and gliomas control nearly every phase of the cell cycle to bolster cellular growth (Alexander, Pinnell, Wen, & D’Andrea, 2012). AEG-1/MTDH/LYRIC has been shown to act as a negative regulator of the BRCA2- and CDKN1A (p21Cip1/Waf-1)-interacting protein, BCCIPα, that binds to the cell cycle regulatory protein, p21, and supports p21-mediated cyclin-dependent kinase 2 (Cdk2) inhibition (Ash, Yang, & Britt, 2008). Without such BCCIPα activity, G1/S cell cycle checkpoint activation following DNA damage is impaired. Moreover, BCCIPα plays a role in homologous recombination after DNA damage (Lu et al., 2005) and contributes to chromosomal stability (Meng, Fan, & Shen, 2007). AEG-1/MTDH/LYRIC physically interacts with BCCIPα, and the amino-terminal domain of AEG-1/MTDH/LYRIC is critical for this interaction. In addition, AEG-1/MTDH/LYRIC overexpression reduces levels of the cyclin-dependent kinase (CDK) inhibitors, p21Cip1 and p27Kip1, as well as phosphorylation of Rb, and enhances progression through the cell cycle following serum starvation (Li et al., 2009). This may occur through regulation of forkhead box-containing O subfamily (FoxO)-1, which acts as a tumor suppressor by transactivating the promoters of CDK inhibitors (Nakamura et al., 2000, Roeb et al., 2007 and Seoane et al., 2004). Knockdown of AEG-1/MTDH/LYRIC reduces the overall percentage of S- and G2/M-phase cells (Liu et al., 2009), which promotes tumor proliferation. AEG-1/MTDH/LYRIC-mediated induction of LEF1 may also control expression of key cell cycle regulatory molecules, including c-myc and Cyclin D1 (Yoo, Emdad, et al., 2009) that promote gliomagenesis (Wang et al., 2012). By circumventing checkpoints that control cellular growth, AEG-1/MTDH/LYRIC hijacks cell cycle function and accelerates glioma progression.
AEG-1/MTDH/LYRIC regulates of angiogenesis in malignant brain tumors
One of the hallmark features of glioblastoma is vascular proliferation, which is marked by an increase in overall vascular density as well as microvascular proliferation in the form of glomeruloid vessels that surround areas of necrosis (Louis, 2006). These vessels support accelerated growth through oxygen and nutrient retrieval in glioblastoma. In addition, they promote several pathological clinical features, including blood–brain barrier breakdown and tumoral edema, through an increase in vascular permeability mediated by vascular endothelial growth factor (VEGF). Cell stress events, such as hypoxia and glucose deprivation, which lead to tumor necrosis, are also known triggers for VEGF activation, which promotes cell survival under metabolic stress (Raza et al., 2002).
AEG-1/MTDH/LYRIC has been shown to regulate a variety of pathways involved in angiogenesis and blood vessel remodeling (Fig. 2). AEG-1/MTDH/LYRIC overexpression in fibroblast cells induces expression of angiopoietin-1 (Ang-1), matrix metalloproteinase (MMP)-2, and HIF-1α, and increases microvessel density (Emdad et al., 2009). Similarly, AEG-1/MTDH/LYRIC overexpression in human hepatocellular carcinoma cells upregulates VEGF, placental growth factor, and fibroblast growth factor-α (Yoo, Emdad, et al., 2009). In addition, AEG-1/MTDH/LYRIC promotes tube formation in cultured endothelial cells, while its knockdown prevents neovascularization in a chicken chorioallantoic membrane model of angiogenesis. As with cellular signaling pathways that promote the AEG-1/MTDH/LYRIC-mediated tumorigenic phenotype, the PI3K-Akt pathway is also critical for enhanced angiogenesis, indicating that there are intermediate pathways that regulate these processes in tumor cells. On a histological level, AEG-1/MTDH/LYRIC is positively associated with expression patterns of molecules regulating invasion, such as MMP-2 and MMP-9, as well as the marker of angiogenesis, CD31 (Emdad et al., 2010). The expression of AEG-1/MTDH/LYRIC also correlates with that of VEGF in breast cancer cells (Li et al., 2011).
The targeting of VEGF has been of moderate success in recurrent glioblastoma since the discovery and testing of the monoclonal antibody, bevacizumab, directed against this growth factor (Friedman et al., 2009). Bevacizumab treatment leads to tumor shrinkage and 6-month progression-free survival that exceeds 40%, and this therapy may be useful against radiation necrosis by reducing edema, mass effect, and leakiness of the blood–brain barrier. However, these benefits are only incremental, and monotherapy against angiogenesis is unlikely to modify disease progression. As AEG-1/MTDH/LYRIC targets extracellular matrix regulatory proteins, invasion, and angiogenesis, the use of small-molecular inhibitors of this pathway along with VEGF inhibition may yield greater efficacy against robust proangiogenic signals in glioblastoma. Further, evidence that AEG-1/MTDH/LYRIC promotes both blood–brain barrier leakiness in virus studies and CNS vascular pathology from migraine studies indicates that AEG-1/MTDH/LYRIC may serve as an efficacious target against tumor angiogenesis.
AEG-1/MTDH/LYRIC promotes tumor-specific glucose metabolism and autophagy in glioblastoma
The metabolic phenotype of cancer is one of the key mechanisms regulating the unrestricted growth of tumor cells. Unlike their normal counterparts, tumor cells preferentially undergo aerobic glycolysis for energy metabolism, a phenomenon first described by Warburg in 1956 known as the Warburg effect (Warburg, 1956). As a result, enhanced glycolytic flux allows tumor cells to produce sufficient adenosine triphosphate (ATP) to fulfill metabolic demands and leads to increased glucose consumption, decreased oxidative phosphorylation, and increased lactate production. Further, this relative surge in glycolytic flux spares tumor cells the production of detrimental reactive oxygen species (ROS) from oxidative phosphorylation and also enhances pentose phosphate pathway flux, which yields reducing equivalents in the form of nicotinamide adenine dinucleotide phosphate. Though glycolysis produces much less energy in the form of ATP than oxidative phosphorylation, several glycolytic enzymes are highly active in tumor cells and support enhanced anaerobic glycolysis and energy production. Specifically, the rate-limiting enzyme that catalyzes the final step in glycolysis, pyruvate kinase, is upregulated in tumor cells. Interestingly, tumor cells exclusively express the M2 isoform of this enzyme (M2PK) (Mazurek, Boschek, Hugo, & Eigenbrodt, 2005), which is typically expressed only during embryonic development, rather than the M1 isoform, expressed in most normal adult tissues. Microarray analysis in hepatocellular carcinoma cells shows that AEG-1/MTDH/LYRIC upregulates pyruvate kinase (Yoo, Emdad, et al., 2009). Additionally, AEG-1/MTDH/LYRIC is a key downstream molecule activated by cell stress, and specifically, is upregulated by hypoxia and glucose deprivation in glioblastoma cells in a ROS-dependent manner (Noch, Bookland, & Khalili, 2011). Moreover, AEG-1/MTDH/LYRIC overexpression allows glioblastoma cells to survive periods of glucose deprivation, whereas its downregulation hastens cell death.
At the center of a host of metabolic pathways is the 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK), which controls the usage of macromolecular precursors for cellular metabolism. AMPK promotes anabolic processes and the Warburg effect in cancer through induction of various glycolytic enzymes. In glioblastoma cells, AEG-1/MTDH/LYRIC reduces the ATP/AMP ratio and activates AMPK and its downstream targets (Bhutia et al., 2010), implicating AEG-1/MTDH/LYRIC as a glycolytic regulator (Fig. 2). In this way, AEG-1/MTDH/LYRIC may enhance glycolytic flux in glioblastoma cells by sensing the cellular energy state and signaling through AMPK to induce elevated levels of PKM2, thereby maintaining sufficient ATP production for tumor proliferation. Further, AEG-1/MTDH/LYRIC may indirectly regulate cellular metabolism through various signaling cascades, such as PI3K/Akt, Wnt/B-catenin, and MAPK/ERK, which all occupy central positions in the oncometabolome and the regulation of oxidative stress. An example is MAPK, which is activated by AEG-1/MTDH/LYRIC and functions in oncogene-driven and oxidative stress-promoted tumorigenesis as well as the induction of glucose transporter expression (Fujishiro et al., 2001).
AMPK is also critical to the process of autophagy, which allows tumor cells to recycle key metabolic end products for future use. In immortalized primary human fetal astrocytes, AEG-1/MTDH/LYRIC promotes autophagy in an AMPK-dependent manner (Bhutia et al., 2010). This is evident with either genetic knockdown of AMPK or pharmacological inhibition. With AEG-1/MTDH/LYRIC overexpression, cellular ATP levels decrease in a dose- and time-dependent manner. However, when cellular ATP stores are replenished, this AEG-1/MTDH/LYRIC-dependent autophagy is reversed, indicating that AEG-1/MTDH/LYRIC acts in this pathway to salvage metabolic intermediates. In normal cells, AEG-1/MTDH/LYRIC promotes autophagy to protect these cells from apoptosis, lending support to a prosurvival function for AEG-1/MTDH/LYRIC in autophagy. In atg5-deficient glioblastoma cells, AEG-1/MTDH/LYRIC overexpression rescues the chemoresistant phenotype, confirming that AEG-1/MTDH/LYRIC-mediated chemoresistance is mediated through autophagy in this context.
On a molecular level, the AEG-1/MTDH/LYRIC-mediated process of autophagy is independent of canonical signaling through beclin 1 and its partner, class III PI3K. Rather, this cascade proceeds through an autophagy protein 5 (ATG5)-dependent pathway through inhibition of mammalian target of rapamycin (mTOR). mTOR is inactivated by AMPK during periods of metabolic stress, which leads to increased ATG5 expression and subsequent autophagy. This complex signaling pathway involving AEG-1/MTDH/LYRIC, AMPK, and mTOR is able to sense the energy status of the cell and the metabolic profile within the tumor microenvironment to coordinately regulate tumor glucose utilization. In this model, AEG-1/MTDH/LYRIC was also shown paradoxically not to increase the production of ROS, which typically induces protective autophagy. Given the role of AEG-1/MTDH/LYRIC in stromal communication, AEG-1/MTDH/LYRIC may also induce the “Reverse Warburg Effect” in cancer, whereby transformed cells utilize oxidative stress to promote autophagy in surrounding stromal cells, thereby providing metabolic precursors that can be shuttled back to cancer cells to promote accelerated growth (Martinez-Outschoorn et al., 2011, Martinez-Outschoorn et al., 2011, Martinez-Outschoorn et al., 2010 and Sotgia et al., 2011). As a result, AEG-1/MTDH/LYRIC may activate both intrinsic and extrinsic autophagy pathways that both promote tumor cell survival and proliferation.
The role of AEG-1/MTDH/LYRIC in tumor invasion and migration
Unlike other soft-tissue masses that exhibit unlimited expansive capacity, brain tumors are restricted by the skull case in which they grow and must either destroy surrounding cells or invade areas of lower cellular density to expand. Gliomas are well known to intercalate through surrounding intercellular crevices through the regulation of chloride flux (Haas and Sontheimer, 2010 and Ransom et al., 2001), autocrine and paracrine chemokine signaling (Hoelzinger et al., 2007 and Wick et al., 2001), and proangiogenic cues. Growth along white matter tracts, around neurons, along blood vessels, and beneath pial margins is typical of glioma, which permits satellite expansion and distant propagation (Louis, 2006 and Scherer, 1938). Invasion through surrounding brain parenchyma is accomplished through the dissolution of extracellular matrix and the activity of various proteases, such as MMPs, a disintegrin and metalloproteinase (ADAM), and ADAMs with thrombospondin motifs (Mentlein et al., 2012 and Rubenstein and Kaufman, 2008). These proteases promote the degradation of microarchitectural scaffolds within normal brain and support oncogenic chemokine and growth factor signaling.
AEG-1/MTDH/LYRIC regulates tumor invasion through a variety of downstream signaling cascades (Fig. 2). AEG-1/MTDH/LYRIC overexpression upregulates genes associated with invasion, such as claudin 4 and tetraspanin 8, and downregulates transgrelin, a suppressor of MMP-9, which together promote adhesion. AEG-1/MTDH/LYRIC overexpression supports the invasive phenotype in neuroblastoma cells (Liu et al., 2010), whereas AEG-1/MTDH/LYRIC knockdown inhibits invasion and decreases MMP-2 and MMP-9 activation through gel zymography in glioma cells. AEG-1/MTDH/LYRIC also activates the MMP-9 promoter (Liu et al., 2010), while AEG-1/MTDH/LYRIC knockdown depresses MMP-2 and MMP-9 promoter activity, indicating a transcriptional regulatory role for AEG-1/MTDH/LYRIC in tumor invasion and adhesion. Similar to transactivation of other promoters, this regulation appears to depend on NFκB, indicating that AEG-1/MTDH/LYRIC may serve as a cooperative regulator of NFκB promoter regulation. These changes in MMP transcription and activation translate to decreased growth of mouse intracranial glioma xenografts, highlighting these pathways as critical to the proliferation and expansion of glioma cells in the brain.
Through coordinated control with lipopolysaccharide (LPS), the main component of the outer membrane of Gram-negative bacteria, AEG-1/MTDH/LYRIC also promotes invasion in malignant brain tumors. Despite a well-known role of LPS in mediating septic shock through the TLR4 (Poltorak et al., 1998), LPS has been implicated in tumor invasion through NFκB pathway activation. In addition, TLR4 activation can lead to enhanced migration and invasion in glioma (Thuringer et al., 2011) and enhances LPS-induced cytokine production (Carpenter et al., 2009). AEG-1/MTDH/LYRIC is induced by LPS through TLR-4 signaling and NFκB phosphorylation (Khuda et al., 2009 and Zhao et al., 2011) and is required for LPS-mediated tumor migration and invasion. The production of IL-8 and MMP9 is reduced by AEG-1/MTDH/LYRIC knockdown but is rescued by LPS treatment. AEG-1/MTDH/LYRIC also regulates expression of the LPS receptor, TLR4, as well as TIR domain-containing adaptor inducing IFN-β (TRIF), within the TLR4 signaling pathway. By enhancing the production of tissue-destructive cytokines, such as MMP9, LPS induction of AEG-1/MTDH/LYRIC fosters increased tumor growth through synergistic TLR4 activation.
In light of the importance of invasion to the process of tumor metastasis, MTDH/AEG-1/MTDH/LYRIC has been demonstrated to induce greater chemotaxis and migration in hepatocellular carcinoma cells, highlighting the strength of this protein in promoting the aggressively invasive phenotype in cancer (Zhou et al., 2012). Further, AEG-1/MTDH/LYRIC induces adhesion of breast cancer cells to the endothelium (Brown and Ruoslahti, 2004 and Hu et al., 2009), which represents a critical component of perivascular spread in glioma (Scherer, 1938). Moreover, AEG-1/MTDH/LYRIC knockdown reduces expression of molecules that support the metastatic phenotype, including N-cadherin and snail, and reduces pulmonary and abdominal metastases (Zhu et al., 2011). N-cadherin is a key molecule mediating cell–cell adhesion and regulates tumor migration and invasion (Gravdal et al., 2007 and Hazan et al., 2000), and therefore, AEG-1/MTDH/LYRIC may control tumor progression through an N-cadherin-dependent mechanism. In glioblastoma, cytoplasmic polyadenylation element-binding protein 1 (CPEB1), which controls the fate of mRNA precursors through the 3′-polyadenylation of target mRNAs, binds to MTDH/AEG-1/MTDH/LYRIC and regulates its expression (Kochanek & Wells, 2013). As CPEB1 modulates directed cellular migration, potentially through trafficking of mature mRNA molecules to the leading edge of dividing cells, AEG-1/MTDH/LYRIC mRNA binding may influence the local expression of AEG-1/MTDH/LYRIC and could implicate this pathway in tumor cellular polarity during invasion. Further, given that CPEB1 shuttles between the nucleus and cytoplasm, this protein may be responsible for context-dependent subcellular localization of AEG-1/MTDH/LYRIC, which carries prognostic importance for patients (Thirkettle, Girling, et al., 2009).
The function of AEG-1/MTDH/LYRIC in RNA interference in malignant glioma
RNA interference serves a pivotal role in regulating the expression of a multitude of human genes and has been utilized for experimental manipulation since its discovery in Caenorhabditis elegans ( Fire et al., 1998). In tumor biology, microRNAs (miRs) act as tumor suppressors and/or oncogenes and modulate tumor cellular processes. The RNA-induced silencing complex (RISC), which facilitates mRNA silencing through association with mature miRNA molecules and assistor proteins, is itself an important mediator of tumorigenesis through associated miRs that can promote cancer ( Moser & Fritzler, 2010). AEG-1/MTDH/LYRIC interacts with several integral proteins that form the RISC complex, including staphylococcal nuclease domain-containing 1 (SND1), ribosomal protein L4 (RPL4), and nucleophosmin 1 (NPM1) ( Blanco et al., 2011, Meng et al., 2012 and Yoo et al., 2011) ( Fig. 2). In addition, AEG-1/MTDH/LYRIC itself is a component of the RISC complex and colocalizes in the cytoplasm not just with SND1 but also with another major RISC component, Ago2 ( Hutvagner & Simard, 2008). AEG-1/MTDH/LYRIC overexpression enhances RISC activity, whereas its knockdown reduces activity in both hepatocellular carcinoma and glioblastoma cells. SND1 inhibition abrogates AEG-1/MTDH/LYRIC function as well, highlighting the tumorigenic capacity of this protein. Interestingly, the region of AEG-1/MTDH/LYRIC that is required for SND1 binding (aa 101–205) is the same region responsible for NFκB p65 (aa 72–169) and BCCIPα interaction, though a specific protein domain has not been identified in this area. As a result, AEG-1/MTDH/LYRIC may utilize a similar binding motif with p65 protein recruitment to traffic other proteins to the RISC complex for cleavage activity.
In this role, AEG-1/MTDH/LYRIC may also regulate the stability and function of miRs involved in tumor activity. AEG-1/MTDH/LYRIC overexpression in glioma cells reduces the tumor-suppressive activity of miR-136, which has been found to be downregulated in human glioma (Yang et al., 2012). Interestingly, miR-136 directly targets the 3′-UTR of AEG-1/MTDH/LYRIC, indicating a potential feedback loop whereby AEG-1/MTDH/LYRIC directs the degradation of a potent suppressor miRNA that would limit its oncogenic function. AEG-1/MTDH/LYRIC suppression is also a result of miR375 activity in head and neck squamous cell carcinoma and esophageal carcinoma (Isozaki et al., 2012 and Nohata et al., 2011).
A further regulatory of AEG-1/MTDH/LYRIC in the processing of mature RNA molecules comes from the study of stress granules. Stress granules are cytoplasmic RNA–protein complexes that are formed when the translation initiation process is halted in response to cellular stress (Buchan & Parker, 2009). AEG-1/MTDH/LYRIC knockdown accentuates the formation of stress granules in response to heat shock and may be responsible for the sequestration of tumor suppressor mRNA during cellular stress (Meng et al., 2012). By blocking the cellular response to stress signals through inhibition of tumor suppressor function, AEG-1/MTDH/LYRIC may encourage aberrant cellular response to these signals, such as impaired DNA repair, redirected metabolism, and enhanced migration and invasion. A more thorough investigation of the RNA-binding activity of AEG-1/MTDH/LYRIC along with its function in RNAi and stress granule formation may facilitate the development of treatments that target these pathways.
The promotion of chemoresistance by AEG-1/MTDH/LYRIC in malignant brain tumors
A critical detriment to the effective treatment of malignant brain tumors is the lack of validated targets, the rapid and inevitable resistance to chemotherapeutic agents, and the difficulty in traversing and achieving therapeutic concentrations within the blood–brain barrier. In malignant gliomas, the chemoresistant phenotype has been studied in relation to a variety of pathways, including antiapoptotic pathways, drug efflux pumps, multidrug resistant genes, and cancer stem cell activity (Lu & Shervington, 2008). The ATP-binding cassette (ABC) superfamily composes one of the largest protein families that is responsible for chemoresistance in glioblastoma (Benyahia et al., 2004 and Spiegl-Kreinecker et al., 2002). The ABC family members transport molecules across cell membranes in an ATP-dependent fashion or through the formation of membrane channels and are expressed in both endothelial cells at the blood–brain barrier and in glioma cells (Bronger et al., 2005). The multidrug resistance gene-1 (MDR-1) protein is overexpressed in high-grade versus low-grade astrocytomas (Kirches et al., 1997 and Spiegl-Kreinecker et al., 2002), contributes to chemoresistance by cancer stem cells (Nakai et al., 2009), and is responsible for treatment outcomes with standard temozolamide chemotherapy (Schaich et al., 2009). Therefore, a better understanding of these proteins and their mechanism of regulation will restrict cellular mechanisms of resistance, such as drug metabolism and efflux that limit therapeutic efficacy.
In addition to its role in promoting tumorigenesis, AEG-1/MTDH/LYRIC also serves an important role in each of these pathways and induces the chemoresistant phenotype (Fig. 2). Most in vitro models have utilized methods of AEG-1/MTDH/LYRIC downregulation to study the therapeutic benefit in promoting sensitivity to chemotherapeutic agents. In a model of hepatocellular carcinoma, AEG-1/MTDH/LYRIC was shown to potently activate dihydropyridine dehydrogenase (DPYD), which converts 5-fluorouracil (5-FU) to its inactive metabolite, fluro-5,6-dihydro uracil (FUH2) ( Yoo, Gredler, et al., 2009). In addition, in this study, AEG-1/MTDH/LYRIC induced expression of thymidylate synthase, which encodes the rate-limiting enzyme in the production of dTTP. AEG-1/MTDH/LYRIC also promotes expression of drug-metabolizing enzymes as well as ABC members, which causes efflux of various chemotherapeutic agents ( Yoo, Emdad, et al., 2009). In glioma cells, AEG-1/MTDH/LYRIC knockdown promotes chemosensitivity ( Emdad et al., 2010) and enhances translation and stability of MDR-1 ( Yoo et al., 2010). Functionally, AEG-1/MTDH/LYRIC promotes chemoresistance to the DNA-alkylating agent, doxorubicin, in an MDR-1-dependent manner and promotes doxorubicin efflux. Moreover, AEG-1/MTDH/LYRIC knockdown in conjunction with treatment using doxorubicin significantly decreases growth of tumor xenografts, indicating a potential therapeutic target to slow tumor growth. Similarly, downregulation of AEG-1/MTDH/LYRIC sensitizes neuroblastoma cells to cisplatin and doxorubicin ( Liu et al., 2009), indicating that AEG-1/MTDH/LYRIC is responsible for chemoresistance in neural crest-derived neoplasms.
AEG-1/MTDH/LYRIC also interacts with multiple oncogenic proteins that are known to mediate chemoresistance in other cancer cell types. In a model of breast cancer, MTDH knockdown was shown to significantly enhance the cytotoxic effects of the potent oral inhibitor of MEK1/2 kinase, selumetinib (AZD6244), possibly through the release of FOXO3a from cytoplasmic proteasomal degradation (Kong, Moran, Zhao, & Yang, 2012). These results emphasize the regulation of protein stability by AEG-1/MTDH/LYRIC and highlight a potential activity that would be suitable for therapeutic intervention.
Metabolic inhibitors, such as 2-deoxyglucose and lonidamine, are also being examined either alone or in combination with traditional chemotherapeutic agents for the treatment of malignant brain tumors (El Mjiyad, Caro-Maldonado, Ramirez-Peinado, & Munoz-Pinedo, 2011). The prevention of metabolic stress by these tumors is a primary strategy to survive periods of cell stress within the tumor microenvironment and to resist antimetabolic therapies. AEG-1/MTDH/LYRIC knockdown suppresses expression of aldehyde dehydrogenase 3 family, member A1 (ALDH3A1) in breast cancer cells, which protects against oxidative stress and also promotes the chemoresistant phenotype (Hu et al., 2009). Additionally, AEG-1/MTDH/LYRIC downregulation sensitizes breast cancer cells to combination treatment with TNF-α-related apoptosis-inducing ligand and the histone deacetylase inhibitor, LBH 589 (Meng et al., 2011). Similar to alkylating agent therapy, it is possible that AEG-1/MTDH/LYRIC knockdown may synergize with metabolic inhibitors to elicit greater cytotoxicity in glioblastoma.
Diagnostic utility of AEG-1/MTDH/LYRIC expression and antibody production in malignant brain tumors
Due to the propensity for the sudden clinical presentation of malignant gliomas through a variety of emergent neurological signs and symptoms that indicate early and extensive invasion, there is a greater need for earlier diagnosis with the use of easily procured patient markers. Much work has been completed in this context for systemic cancers, but progress has been disappointing in the field of malignant brain tumors. This relative shortcoming may result from the considerable difficulty in obtaining useful samples from the CNS compartment and/or the inability to identify serum markers that reflect the status of the tumor and its microenvironment within the CNS. Most traditional biomarkers, such as platelet-derived growth factor, epidermal growth factor receptor (EGFR), O6-methylguanine-DNA methyltransferase, and IDH1, must be measured from tumor tissue itself, while many identified serum biomarkers do not correlate with those in cerebrospinal fluid (CSF). In glioblastoma, in particular, some potential biomarkers may be highly expressed in normal brain, making it difficult to differentiate tumor aggressiveness from normal brain processes.
A trove of evidence exists that AEG-1/MTDH/LYRIC correlates with tumor grade in many cancers (Yoo, Emdad, et al., 2011), and similar data have been demonstrated in glioblastoma (Emdad et al., 2010 and Liu et al., 2010). Therefore, it is evident that AEG-1/MTDH/LYRIC expression alone may serve as a stratifying marker for malignant potential. However, this strategy is only useful once tumor tissue is obtained and is not valuable prior to diagnosis or with inoperable or difficult-to-biopsy tumors. Though AEG-1/MTDH/LYRIC is not predominantly localized to the cell membrane, antibodies to this protein have been discovered in the serum of patients with cancer (Chen et al., 2012). Moreover, levels of this antibody correlate with tumor grade and therefore reflect the aggressive nature of the tumor itself. Despite the lack of evidence of these serum antibodies in glioblastoma patients, it is possible that they do exist, if not in the serum, then in the CSF that bathes glioblastoma cells. Identification of these antibodies in brain tumor patients may allow noninvasive longitudinal follow-up that could supplement imaging studies after standard therapeutic paradigms. However, given the propensity for late presentation, the role of early detection in glioblastoma is unclear, other than in select patients who suffer from familial disorders that predispose to the development of malignant brain tumors.
Treatment strategies for malignant brain tumors derived from the AEG-1/MTDH/LYRIC pathway The development of novel therapies for malignant brain tumors is largely based on the validation of targets that have been previously identified. In the field of glioma, few therapies have led to any significant success over median survivals of 12–15 months established with earlier therapies (Stupp et al., 2005). These shortcomings are due to the inability to achieve sufficient therapeutic concentrations within the CNS, to rapid development of chemoresistance, and to the oncogenic mechanisms to subvert disease control with various biologic and nonbiologic treatment strategies.
The discovery of DNA vaccines as a potential strategy to treat cancer has led to their use in a variety of systemic malignancies and multiple clinical trials testing their efficacy (Rice, Ottensmeier, & Stevenson, 2008). As this therapeutic strategy involves the use of a DNA vaccine that is long-lived in vivo and relatively easy to deliver, it may be possible to utilize such therapy for the treatment of malignant brain tumors as well. The appropriate choice for DNA vaccine targets is critical as many tumor antigens are present on normal cells, with a resultant vaccine response yielding either low efficacy or autoimmunity. Given the overexpression of AEG-1/MTDH/LYRIC in human cancer, an AEG-1/MTDH/LYRIC-based, DNA-based vaccine was developed for use in breast cancer. The AEG-1/MTDH/LYRIC-based DNA vaccine inhibits the growth of breast tumors in mice, promotes a robust cytotoxic T cell response, and enhances overall survival ( Qian et al., 2011). Such methods could easily be applied to glioblastoma to assess vaccine efficacy within the CNS. Indeed, DNA vaccines have been effective against well-characterized targets in glioblastoma, such as VEGF, that is critical to angiogenesis ( Niethammer et al., 2002) and have been useful in models of metastatic melanoma ( Pertl et al., 2003). In addition, peptide vaccines specifically targeted against the EGFR have achieved some success in the treatment of glioblastoma in phase I studies (Sampson et al., 2010), with phase II trials currently underway.
It is evident that AEG-1/MTDH/LYRIC knockdown inhibits the growth of glioblastoma and improves overall survival in animal models (Emdad et al., 2010). Knockdown strategies primarily involve the use of RNAi to achieve gene silencing and may also point to therapeutic possibilities in humans. Small-molecule inhibitors of AEG-1/MTDH/LYRIC may be relevant in targeting this protein given its ubiquitous expression in cells and its central role in oncogenic signaling cascades. Moreover, given the intimate interaction of AEG-1/MTDH/LYRIC with other tumorigenic pathways, small-molecule inhibitors of those pathways may also target AEG-1/MTDH/LYRIC. Such evidence has been demonstrated with the use of MMP (Liu et al., 2010) and CPEB inhibition (Kochanek & Wells, 2013). What is not currently known is the role of AEG-1/MTDH/LYRIC in normal cells, and the effects of such targeted therapies against AEG-1/MTDH/LYRIC may lead to significant adverse reactions when investigated in animal models. Further examination of the role of AEG-1/MTDH/LYRIC under normal conditions and a more expansive understanding of its expression pattern in normal brain, in particular, may aid in the development of AEG-1/MTDH/LYRIC-based therapies for malignant brain tumors.
Conclusions
The burden of neurological disease in the human population continues to increase with a relative lack of efficacious treatments that improve patient morbidity and mortality. From migraines to neurodegenerative disease and from infectious diseases to cancer, the CNS remains at the center of concerted efforts in biomedical research, both to understand the pathogenesis of neurological disease and to develop innovative therapies. As the human population ages, the incidence of neurodegenerative disease will increase, and there will be a greater need for disease-modifying therapies. Similarly, without robust targeted treatment strategies that improve patient survival, malignant tumors of the CNS will continue to carry single-digit 5-year survivals. A greater thrust to identify common pathways underlying CNS disease and points along these cascades that are amenable to therapeutic intervention is critical to reducing the devastating consequences of CNS disease.
As a potent oncogene that is central to diverse cellular signaling pathways, AEG-1/MTDH/LYRIC represents an important gene that contributes to the pathogenesis of diseases within the CNS. By promoting glutamate excitotoxicity and reactive gliosis, AEG-1/MTDH/LYRIC contributes to the neurological sequelae of HIV-1 infection. Its association with a migraine susceptibility locus on chromosome 8q22 suggests its involvement with the pathophysiology of vascular disease within the CNS. Perhaps, the best studied of the effects of AEG-1/MTDH/LYRIC with regard to neurological disease, however, is its involvement in CNS tumorigenesis. AEG-1/MTDH/LYRIC coordinates a network of oncogenic signaling cascades that contribute to aggressive growth, migration and invasion, angiogenesis, and tumor-specific metabolism that facilitates rapid expansion. In addition, AEG-1/MTDH/LYRIC overexpression in glioblastoma supports the chemoresistant phenotype and potentiates RNA interference-directed silencing of tumor suppressor genes. In light of its importance in tumorigenesis, mounting evidence suggests that antibodies are generated against AEG-1/MTDH/LYRIC in the serum of cancer patients, and AEG-1/MTDH/LYRIC downregulation using DNA vaccination may prolong overall survival.
At the time of initial cloning and characterization, AEG-1/MTDH/LYRIC was thought to contribute to several distinct but central processes underlying HIV-1 infection, glutamate regulation, and tumorigenesis. It is now clear that AEG-1/MTDH/LYRIC contributes significantly to disease within the CNS and represents a potentially valuable target for therapeutic investigation. Though much is now known regarding the role of this oncogenic protein within the CNS compartment, further examination into its precise mechanism of regulation will facilitate the development of treatments that target this pathway or interrelated processes that govern AEG-1/MTDH/LYRIC activity. With an increasing number of identified and validated targets within the AEG-1/MTDH/LYRIC signaling pathway, it is conceivable that AEG-1/MTDH/LYRIC-specific molecules could exhibit efficacy in the treatment of and improve outcomes in neurological disease.
References
- Alexander BM, Pinnell N, Wen PY, D’Andrea A. Targeting DNA repair and the cell cycle in glioblastoma. Journal of Neuro-Oncology. 2012;107:463–477. doi: 10.1007/s11060-011-0765-4. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Müller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. The Journal of Biological Chemistry. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nature Genetics. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash SC, Yang DQ, Britt DE. LYRIC/AEG-1/MTDH/LYRIC overexpression modulates BCCIPalpha protein levels in prostate tumor cells. Biochemical and Biophysical Research Communications. 2008;371:333–338. doi: 10.1016/j.bbrc.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyahia B, Huguet S, Decleves X, Mokhtari K, Criniere E, Bernaudin JF, et al. Multidrug resistance-associated protein MRP1 expression in human gliomas: Chemosensitization to vincristine and etoposide by indomethacin in human glioma cell lines overexpressing MRP1. Journal of Neuro-Oncology. 2004;66:65–70. doi: 10.1023/b:neon.0000013484.73208.a4. [DOI] [PubMed] [Google Scholar]
- Bernardo MV, Yelo E, Gimeno L, Campillo JA, Parrado A. Identification of apoptosis-related PLZF target genes. Biochemical and Biophysical Research Communications. 2007;359:317–322. doi: 10.1016/j.bbrc.2007.05.085. [DOI] [PubMed] [Google Scholar]
- Bhutia SK, Kegelman TP, Das SK, Azab B, Su ZZ, Lee SG, et al. Astrocyte elevated gene-1 induces protective autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22243–22248. doi: 10.1073/pnas.1009479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MA, Aleckovic M, Hua Y, Li T, Wei Y, Xu Z, et al. Identification of staphylococcal nuclease domain-containing 1 (SND1) as a Metadherin-interacting protein with metastasis-promoting functions. The Journal of Biological Chemistry. 2011;286:19982–19992. doi: 10.1074/jbc.M111.240077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Experimental Cell Research. 2004;300:134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Research. 2005;65:11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Buchan JR, Parker R. Eukaryotic stress granules: The ins and outs of translation. Molecular Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, et al. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. The Journal of Biological Chemistry. 2009;284:18167–18173. doi: 10.1074/jbc.M109.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, Carlson T, Dellacasagrande J, Garcia A, Gibbons S, Hertzog P, et al. TRIL, a functional component of the TLR4 signaling complex, highly expressed in brain. The Journal of Immunology. 2009;183:3989–3995. doi: 10.4049/jimmunol.0901518. [DOI] [PubMed] [Google Scholar]
- Chen X, Dong K, Long M, Lin F, Wang X, Wei J, et al. Serum anti-AEG-1/MTDH/LYRIC auto-antibody is a potential novel biomarker for malignant tumors. Oncology Letters. 2012;4:319–323. doi: 10.3892/ol.2012.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005–2009. Neuro-Oncol. 2012;14(suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253–264. doi: 10.1038/onc.2010.466. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, et al. Astrocyte elevated gene-1 (AEG-1/MTDH/LYRIC) functions as an oncogene and regulates angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: A novel target for human glioma therapy. Molecular Cancer Therapeutics. 2010;9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Research. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- Engeland CE, Oberwinkler H, Schümann M, Krause E, Müller GA, Kräusslich HG. The cellular protein lyric interacts with HIV-1 Gag. Journal of Virology. 2011;85:13322–13332. doi: 10.1128/JVI.00174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, et al. MKK6/3 and p38 MAPK pathway activation is not necessary for insulin-induced glucose uptake but regulates glucose transporter expression. The Journal of Biological Chemistry. 2001;276:19800–19806. doi: 10.1074/jbc.M101087200. [DOI] [PubMed] [Google Scholar]
- Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. The Journal of Cellular Biology. 1997;136:531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: Symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clinical Cancer Research. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- Haas BR, Sontheimer H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 reduces glioma invasion. Cancer Research. 2010;70:5597–5606. doi: 10.1158/0008-5472.CAN-09-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. The Journal of Cell Biology. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. Journal of the National Cancer Institute. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ. Argonaute proteins: Key players in RNA silencing. Nature Reviews Molecular Cell Biology. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Isozaki Y, Hoshino I, Nohata N, Kinoshita T, Akutsu Y, Hanari N, et al. Identification of novel molecular targets regulated by tumor suppressive miR-375 induced by histone acetylation in esophageal squamous cell carcinoma. International Journal of Oncology. 2012;41:985–994. doi: 10.3892/ijo.2012.1537. [DOI] [PubMed] [Google Scholar]
- Jeon HY, Choi M, Howlett EL, Vozhilla N, Yoo BK, Lloyd JA, et al. Expression patterns of astrocyte elevated gene-1 (AEG-1/MTDH/LYRIC) during development of the mouse embryo. Gene Expression Patterns. 2010;10:361–367. doi: 10.1016/j.gep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-bo X, Hui W, Yu-long H, Chang-hua Z, Long-juan Z, Shi-rong C, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Medical Oncology. 2011;28:455–462. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Chang SM. Recent medical management of glioblastoma. Advances in Experimental Medicine and Biology. 2012;746:26–40. doi: 10.1007/978-1-4614-3146-6_3. [DOI] [PubMed] [Google Scholar]
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1/MTDH/LYRIC. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kaur N, Chettiar S, Rathod S, Rath P, Muzumdar D, Shaikh ML, et al. Wnt3a mediated activation of Wnt/beta-catenin signaling promotes tumor progression in glioblastoma. Molecular and Cellular Neurosciences. 2013;54C:44–57. doi: 10.1016/j.mcn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Khuda II, Koide N, Noman AS, Dagvadorj J, Tumurkhuu G, Naiki Y, et al. Astrocyte elevated gene-1 (AEG-1/MTDH/LYRIC) is induced by lipopolysaccharide as toll-like receptor 4 (TLR4) ligand and regulates TLR4 signalling. Immunology. 2009;128:e700–e706. doi: 10.1111/j.1365-2567.2009.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- Kirches E, Oda Y, Von Bossanyi P, Diete S, Schneider T, Warich-Kirches M, et al. Mdr1 mRNA expression differs between grade III astrocytomas and glioblastomas. Clinical Neuropathology. 1997;16:34–36. [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annual Review of Biochemistry. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Kochanek DM, Wells DG. CPEB1 regulates the expression of MTDH/AEG-1/MTDH/LYRIC and glioblastoma cell migration. Molecular Cancer Research. 2013;11:149–160. doi: 10.1158/1541-7786.MCR-12-0498. [DOI] [PubMed] [Google Scholar]
- Kong X, Moran MS, Zhao Y, Yang Q. Inhibition of metadherin sensitizes breast cancer cells to AZD6244. Cancer Biology & Therapy. 2012;13:43–49. doi: 10.4161/cbt.13.1.18868. [DOI] [PubMed] [Google Scholar]
- Lee SG, Jeon HY, Su ZZ, Richards JE, Vozhilla N, Sarkar D, et al. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28:2476–2484. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1/MTDH/LYRIC) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Song H, Wang D, Feng T, Yu X, et al. Significance of AEG-1/MTDH/LYRIC expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. Journal of Surgical Oncology. 2011;103:184–192. doi: 10.1002/jso.21788. [DOI] [PubMed] [Google Scholar]
- Li J, Yang L, Song L, Xiong H, Wang L, Yan X, et al. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene. 2009;28:3188–3196. doi: 10.1038/onc.2009.171. [DOI] [PubMed] [Google Scholar]
- Ligthart L, de Vries B, Smith AV, Ikram MA, Amin N, Hottenga JJ, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. European Journal of Human Genetics. 2011;19:901–907. doi: 10.1038/ejhg.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino MM, Merlo A. PI3Kinase signaling in glioblastoma. Journal of Neuro-Oncology. 2011;103:417–427. doi: 10.1007/s11060-010-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IJ, Chiu CY, Chen YC, Wu HC. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. The Journal of Biological Chemistry. 2011;286:9726–9736. doi: 10.1074/jbc.M110.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Liu CX, Han B, Zhang XY, Sun RP. AEG-1/MTDH/LYRIC is associated with clinical outcome in neuroblastoma patients. Cancer Biomarkers. 2012;11:115–121. doi: 10.3233/CBM-2012-0268. [DOI] [PubMed] [Google Scholar]
- Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. Journal of Experimental & Clinical Cancer Research. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, et al. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Research. 2010;70:3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- Louis DN. Molecular pathology of malignant gliomas. Annual Review of Pathology. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- Lu H, Guo X, Meng X, Liu J, Allen C, Wray J, et al. The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Molecular and Cellular Biology. 2005;25:1949–1957. doi: 10.1128/MCB.25.5.1949-1957.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Shervington A. Chemoresistance in gliomas. Molecular and Cellular Biochemistry. 2008;312:71–80. doi: 10.1007/s11010-008-9722-8. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of disease: Astrocytes in neurodegenerative disease. Nature Clinical Practice Neurology. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Lin Z, Ko YH, Goldberg AF, Flomenberg N, Wang C, et al. Understanding the metabolic basis of drug resistance: Therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10:2521–2528. doi: 10.4161/cc.10.15.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, et al. Stromal-epithelial metabolic coupling in cancer: Integrating autophagy and metabolism in the tumor microenvironment. The International Journal of Biochemistry & Cell Biology. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Casey T, et al. The autophagic tumor stroma model of cancer or “battery-operated tumor growth”: A simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–4306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Seminars in Cancer Biology. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of Neurology. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, et al. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Fan J, Shen Z. Roles of BCCIP in chromosome stability and cytokinesis. Oncogene. 2007;26:6253–6260. doi: 10.1038/sj.onc.1210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhu D, Yang S, Wang X, Xiong Z, Zhang Y, et al. Cytoplasmic Metadherin (MTDH) provides survival advantage under conditions of stress by acting as RNA-binding protein. The Journal of Biological Chemistry. 2012;287:4485–4491. doi: 10.1074/jbc.C111.291518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, Hattermann K, Held-Feindt J. Lost in disruption: Role of proteases in glioma invasion and progression. Biochimica et Biophysica Acta. 2012;1825:178–185. doi: 10.1016/j.bbcan.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Moser JJ, Fritzler MJ. The microRNA and messengerRNA profile of the RNA-induced silencing complex in human primary astrocyte and astrocytoma cells. PLoS One. 2010;5:e13445. doi: 10.1371/journal.pone.0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai E, Park K, Yawata T, Chihara T, Kumazawa A, Nakabayashi H, et al. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Investigation. 2009;27:901–908. doi: 10.3109/07357900801946679. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Molecular and Cellular Biology. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nature Medicine. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- Noch E, Bookland M, Khalili K. Astrocyte-elevated gene-1 (AEG-1/MTDH/LYRIC) induction by hypoxia and glucose deprivation in glioblastoma. Cancer Biology & Therapy. 2011;11:32–39. doi: 10.4161/cbt.11.1.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biology & Therapy. 2009;8:1791–1797. doi: 10.4161/cbt.8.19.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, et al. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH/LYRIC/MTDH in head and neck squamous cell carcinoma (HNSCC) Journal of Human Genetics. 2011;56:595–601. doi: 10.1038/jhg.2011.66. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Science Signaling. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Pertl U, Wodrich H, Ruehlmann JM, Gillies SD, Lode HN, Reisfeld RA. Immunotherapy with a posttranscriptionally modified DNA vaccine induces complete protection against metastatic neuroblastoma. Blood. 2003;101:649–654. doi: 10.1182/blood-2002-02-0391. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Purow B, Schiff D. Advances in the genetics of glioblastoma: Are we reaching critical mass? Nature Reviews Neurology. 2009;5:419–426. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BJ, Yan F, Li N, Liu QL, Lin YH, Liu CM, et al. MTDH/AEG-1/MTDH/LYRIC-based DNA vaccine suppresses lung metastasis and enhances chemosensitivity to doxorubicin in breast cancer. Cancer Immunology, Immunotherapy. 2011;60:883–893. doi: 10.1007/s00262-011-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, O’Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21:7674–7683. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: A friend or a foe? A review and a hypothesis. Neurosurgery. 2002;51:2–12. doi: 10.1097/00006123-200207000-00002. discussion 12–13. [DOI] [PubMed] [Google Scholar]
- Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: Precision tools for activating effective immunity against cancer. Nature Reviews Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Research. 2001;61:3556–3560. [PubMed] [Google Scholar]
- Roeb W, Boyer A, Cavenee WK, Arden KC. PAX3-FOXO1 controls expression of the p57Kip2 cell-cycle regulator through degradation of EGR1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18085–18090. doi: 10.1073/pnas.0708910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurology. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- Rubenstein BM, Kaufman LJ. The role of extracellular matrix in glioma invasion: A cellular Potts model approach. Biophysical Journal. 2008;95:5661–5680. doi: 10.1529/biophysj.108.140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri F, Titanji K, De Milito A, Chiodi F. Astrocyte activation and apoptosis: Their roles in the neuropathology of HIV infection. Brain Pathology. 2003;13:84–94. doi: 10.1111/j.1750-3639.2003.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. The Journal of Cell Biology. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Research. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- Schaich M, Kestel L, Pfirrmann M, Robel K, Illmer T, Kramer M, et al. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2009;20:175–181. doi: 10.1093/annonc/mdn548. [DOI] [PubMed] [Google Scholar]
- Scherer H. Structural development in gliomas. American Journal of Cancer. 1938;34:333–351. [Google Scholar]
- Schulz M, Brandner S, Eberhagen C, Eckardt-Schupp F, Larsen MR, Andrae U. Quantitative phosphoproteomic analysis of early alterations in protein phosphorylation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of Proteome Research. 2013;12:866–882. doi: 10.1021/pr3009429. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Lisanti MP. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Research. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegl-Kreinecker S, Buchroithner J, Elbling L, Steiner E, Wurm G, Bodenteich A, et al. Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. Journal of Neuro-Oncology. 2002;57:27–36. doi: 10.1023/a:1015735815111. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Chen Y, Kang DC, Chao W, Simm M, Volsky DJ, et al. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-alpha treatment. Gene. 2003;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: A novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Experimental Cell Research. 2004;294:94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H, et al. LYRIC/AEG-1/MTDH/LYRIC is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clinical Cancer Research. 2009;15:3003–3013. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- Thirkettle HJ, Mills IG, Whitaker HC, Neal DE. Nuclear LYRIC/AEG-1/MTDH/LYRIC interacts with PLZF and relieves PLZF-mediated repression. Oncogene. 2009;28:3663–3670. doi: 10.1038/onc.2009.223. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Hammann A, Benikhlef N, Fourmaux E, Bouchot A, Wettstein G, et al. Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. The Journal of Biological Chemistry. 2011;286:3418–3428. doi: 10.1074/jbc.M110.154823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak-Sharma N, Ghorpade A. Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: Implications for reactive astrogliosis and neurodegeneration. Journal of Neuroinflammation. 2012;9:195. doi: 10.1186/1742-2094-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Q, Cui Y, Liu ZY, Zhao W, Wang CL, et al. Knockdown of cyclin D1 inhibits proliferation, induces apoptosis, and attenuates the invasive capacity of human glioblastoma cells. Journal of Neuro-Oncology. 2012;106:473–484. doi: 10.1007/s11060-011-0692-4. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science (New York, NY) 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wick W, Platten M, Weller M. Glioma cell invasion: Regulation of metalloproteinase activity by TGF-beta. Journal of Neuro-Oncology. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- Xia Z, Zhang N, Jin H, Yu Z, Xu G, Huang Z. Clinical significance of astrocyte elevated gene-1 expression in human oligodendrogliomas. Clinical Neurology and Neurosurgery. 2010;112:413–419. doi: 10.1016/j.clineuro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, et al. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1/MTDH/LYRIC and Bcl-2. FEBS Letters. 2012;586:3608–3612. doi: 10.1016/j.febslet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, et al. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Research. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, et al. Astrocyte elevated gene-1 (AEG-1/MTDH/LYRIC): A multifunctional regulator of normal and abnormal physiology. Pharmacology & Therapeutics. 2011;130:1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. The Journal of Clinical Investigation. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, et al. Identification of genes conferring resistance to 5-fluorouracil. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology. 2011;53:1538–1548. doi: 10.1002/hep.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Li X, Yan S, Yang Q, Liu X, Kong B. The MTDH (− 470G > A) polymorphism is associated with ovarian cancer susceptibility. PLoS One. 2012;7:e51561. doi: 10.1371/journal.pone.0051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, et al. Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS One. 2011;6:e29363. doi: 10.1371/journal.pone.0029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Deng H, Yan W, Huang H, Deng Y, Li Y, et al. Expression of metadherin/AEG-1/MTDH/LYRIC gene is positively related to orientation chemotaxis and adhesion of human hepatocellular carcinoma cell lines of different metastatic potentials. Journal of Huazhong University of Science and Technology Medical Sciences. 2012;32:353–357. doi: 10.1007/s11596-012-0061-3. [DOI] [PubMed] [Google Scholar]
- Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clinical Cancer Research. 2011;17:7294–7302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]