Fig. 5.
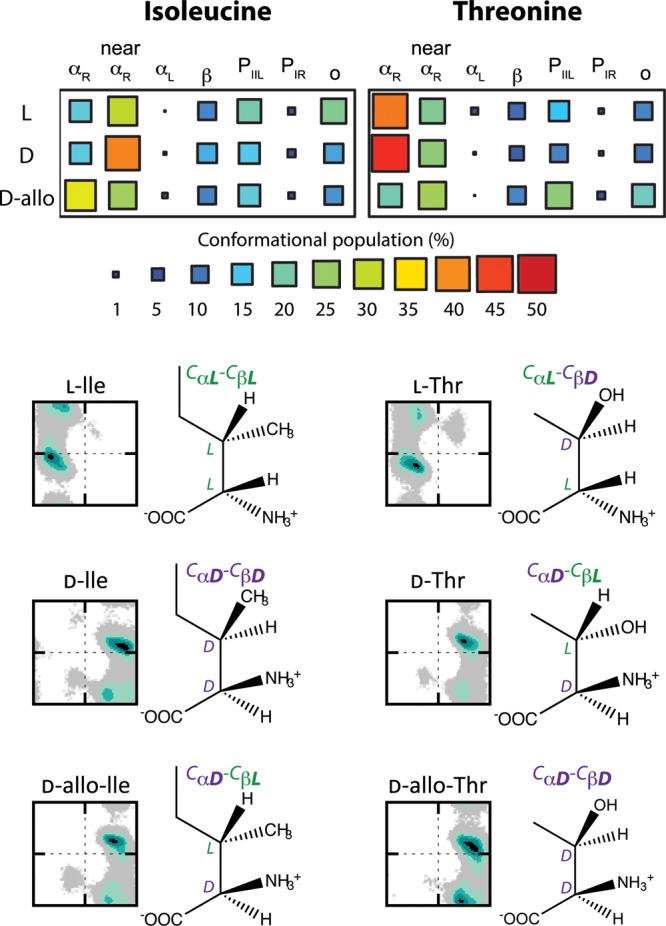
Differences in the conformational propensities of three enantiomers of isoleucine and threonine possible due to the additional chiral center in the side chains. d-Ile and d-Thr are true reflections of the naturally occurring l-Ile and l-Thr enantiomers. The allo-forms, d-allo-isoleucine and d-allo-threonine, have been observed from post-translational modifications involving epimerization at the Cα position; only the stereochemistry at the backbone is affected with that of the side chains remaining the same. The percentage population of specified conformational regions is shown along with the corresponding Ramachandran plots demonstrating the impact of the alternative stereochemistry in the side chain on the sampling of the d-amino acids. For the d-forms, the conformational regions are those inverted across the glide plane to correspond with the same areas specified for the l-forms as shown in Figs. 1A and B.
