Abstract
Background/Aims
Ethanol administration causes intestinal epithelial cell damage by increasing intestinal permeability and the translocation of endotoxins from intestinal bacterial flora. Heat shock proteins (HSPs) are associated with recovery and protection from cell damage. The aim of the current study was to investigate differences in the expression of HSPs in the small intestine and the biochemical changes attributable to ethanol-induced intestinal damage.
Methods
Ethanol (20%) was injected intraperitoneally (2.75 g/kg, 5.5 g/kg, 8.25 g/kg) in ICR mice and the same volume of saline was administered to controls. After 1 hour, the proximal, middle, and distal segments were taken from the small intestine and the degree of damage was analyzed. In each segment, the expression of HSPs was analyzed by western blotting. The expression of inflammatory mediators including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), and antioxidant enzyme such as glutathione-S-transferase were compared using real-time polymerase chain reaction assays.
Results
In the control group, HSP70 increased in all segments of small intestine. Additionally, increases in the expression of HSP40 and HSP90 in the distal regions and an increase in HSP32 in the middle regions were observed. After ethanol treatment, greater histological damage was observed in the distal small intestine and significant decreases in HSPs were observed generally. Increased expression of IL-1β, TNF-α, and COX-2 was observed in small intestinal tissues exposed to ethanol-induced damage. However, there was no significant difference in the expression of an antioxidant enzyme.
Conclusions
Significant differences in the expression of HSPs in different intestinal regions were observed. These differences may have been attributable to the distribution of intestinal bacteria.
Keywords: Intestine, small; Heat shock protein; Ethanol
INTRODUCTION
A variety of structural and functional abnormalities are caused by acute and chronic exposure to ethanol. More specifically, acute administration of ethanol damages epithelial cells of the mucosa in the small intestine, increases intestinal permeability, and induces the translocation of endotoxins. Furthermore, it also impairs active transport mechanisms and inhibits the absorption of various nutrients, including vitamins. Despite ongoing research, the exact pathological mechanisms underlying damage caused by ethanol exposure in the small intestine remain unclear.1,2
Several reports have indicated that the structural and functional abnormalities in the small intestine following exposure to alcohol may be related to oxidative stress which was suggested as a possible mechanism for the observed ethanol-induced damage to increase intestinal permeability.3 On the other hand, oxidative stress may induce the synthesis of heat shock proteins (HSPs), cellular structural proteins known to play a central role in the elimination and modification of denatured cellular proteins and the prevention of aggregation of those proteins. They function as molecular chaperones that mediate protein synthesis, folding, transport, translocation, and processing.4 Based on their molecular weight, they have been classified into the HSP60, HSP70, and HSP90 families, as well as the small HSP family. Several reports have addressed the roles of HSPs in intestinal inflammation, however, few investigations regarding the effects of HSPs in ethanol-induced damage to the small intestine have been conducted.5
In addition to increased intestinal permeability, bacterial flora may be related to intestinal damage. Bacterial flora present in the gastric intestinal tract are present at a level of 103 colony-forming units (CFU)/mL or less in the stomach but can be increased up to 102-103 bacteria/mL, 108 bacteria/mL, and 1012 bacteria/mL in the proximal jejunum, distal ileum, and colon, respectively.6 Furthermore, the composition of bacterial flora changes gradually from gram-positive to gram-negative from the proximal to the distal end. As the composition and density of bacterial flora may vary along the length of the small intestine, one might expect that the degree and mechanisms underlying their pathological damage may be different as well.
Therefore, the current study was conducted to examine pathological changes in different regions of the small intestine induced by ethanol exposure in order to investigate underlying mechanisms. Furthermore, the association between oxidative stress and small intestine damage was analyzed by assessing antioxidative levels and inflammatory factors. Lastly, changes in the expression of HSPs, which are regulatory factors for cellular oxidative damage, were also examined.
METHODS
1. Experimental Animals and Ethanol Injection
In the current study, specific-pathogen-free (SPF) 8-week-old male ICR mice were obtained from Dae Han Biolink Co. (Eumsung, Korea) and housed in specific SPF chambers at the School of Medicine in Kangwon National University. The SPF chambers in the animal room were maintained at 23±1℃ and 55±7% relative humidity and mice were acclimated for 3 days prior to the experiments. Animals had free access to water and diets and were housed on a 12:12 hour light-dark cycle. All experiments were approved by the Animal Experiment Ethics Committee of Kangwon National University in advance of the study (Approval Number: KIACUC-11-0012).
In the experiment, mice were randomly allocated into 4 groups: a control group (phosphate buffered saline [PBS] treated), and 3 ethanol groups (2.75 g/kg, 5.5 g/kg, and 8.25 g/kg). Ethanol (Merck, Damstadt, Germany) was diluted with saline (PBS) and then administered via intraperitoneal injection. Small intestines were harvested 1 hour after the injection.
2. Macroscopic Assessment and Histological Analyses
ICR mice were administered either PBS (control group) or PBS diluted anhydrous ethanol (2.75 g/kg, 5.5 g/kg, or 8.25 g/kg) via intraperitoneal injection. Subsequently, alcohol-induced damage to the small intestines was analyzed. The small intestine was divided into 3 parts: the proximal jejunum, the middle of the small intestine, and the distal ileum. Each tissue segment with the size of 3 cm was collected and fixed with formalin solution and then histological changes were observed via pathological examinations using optical microscopy (Olympus BX43; Olympus Co., Tokyo, Japan).
3. RNA Analysis
1) RNA Extraction
One-hour after the intraperitoneal injection of ethanol, small intestines were harvested from ICR mice and total genomic RNA was extracted using the easy-spin Total RNA Extraction Kit (Intron, Seongnam, Korea) according to the manufacturer's instructions. Quantitative and qualitative analyses of the extracted RNA were conducted using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). RNA samples were stored at -70℃ until further analysis.
2) The Synthesis of cDNA and Real-time PCR Analysis
The cDNA was synthesized using extracted total RNA (2 µg), 2.5 mM oligo (dt), and an Omniscript RT kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions in order to monitor changes in mRNA expression of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and cyclooxygenase-2 (COX-2) (GeneAmp PCR system 2700; Applied Biosystems, Foster City, CA, USA). The primers for each gene were obtained from Bioneer Corp. (Daejeon, Korea) and base sequences of primers are summarized in Table 1. The respective primer pair for each gene was added to a mixture (20 µL) of synthesized cDNA, 1× Rox reference Dye (Takara Bio, Otsu, Shiga, Japan), and 1× STBR premix Ex Tag (Takara Bio, Otsu, Shiga, Japan). Real-time PCR reactions were performed using the Applied Biosystems 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Changes in the expression level of each gene were applied into the -2ΔΔCt equation using a housekeeping gene (i.e., glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) and then corrected. Corrected values were compared to those of the PBS-treated control group (ΔCt=gene of interest Ct-GAPDH Ct, -ΔΔCt=ΔCt treated-ΔCt control).
Table 1.
List of Primers Used for Real-time PCR
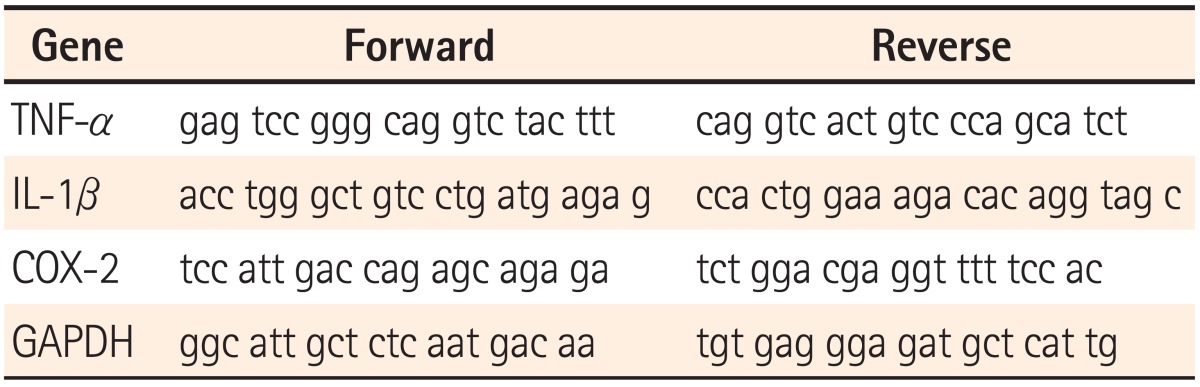
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; COX-2, cyclooxygenase-2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
4. Protein Analysis
One-hour after the intraperitoneal injection of ethanol, small intestines were harvested from ICR mice and protein was extracted using the PRO-PREP Kit (Intron, Seongnam, Korea) per the manufacturer's instructions.7 Protein quantification was conducted using the BCA (Sigma, St. Louis, MO, USA) protein quantification reagent. Western blotting was performed in order to analyze protein samples. Protein samples were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. Once transferred, polyvinylidene fluoride membranes were blocked with 5% blocking buffer (i.e., non-fat dry milk) for 30 minutes and then incubated with primary antibody diluted into blocking buffer for 16 hours at 4℃ (HSP40, HSP70, and HSP90 antibody, 1:1,000, Stressgen, Ann Arbor, MI, USA; HSP32 [heme oxygenase-1, HO-1] antibody, 1:1,000, Abcam, Cambridge, MA, USA; glutathione-S-transferase [GST] antibody, 1:1,000, Sigma, St. Louis, MO, USA; β-actin antibody, 1:2,500, Sigma, St. Louis, MO, USA). The membrane was washed 3 times for 10 minutes and then incubated with secondary antibody for 2 hours at room temperature (horseradish peroxidase-conjugated goat anti-rabbit IgG, horseradish peroxidase-conjugated goat anti-mouse IgG, 1:2,000, Jackson ImmunoResearch, West Grove, PA, USA). Once incubated, the membrane was washed with TBST 5 times and then incubated again with ECL (Amersham, Pittsburgh, PA, USA) in advance of exposure against X-ray film (Kodak, Rochester, NY, USA). Developed protein bands on the film were analyzed using the Image Quant software (Molecular Dynamics, Sunnyvale, CA, USA) and then corrected with a housekeeping gene (β-actin). Proteins were extracted from the respective segments of the small intestine and then quantified once. The expression of the respective proteins was analyzed in triplicate.
5. Statistical Analysis
The statistical significance of the results was analyzed via the non-parametric Kruskal-Wallis test and Mann-Whitney U test post-hoc analysis (SPSS version 12.0, SPSS, Inc., Chicago, IL, USA). A P-value<0.05 was considered as statistically significant.
RESULTS
1. Determination of the Time Point for Harvesting Small Intestines Following the Administration of Ethanol
In the current study, the small intestines were dissected following 1 hour of ethanol administration. This was based on the results of a preliminary study which tested various time points (i.e., 30 minutes, 1 hour, 3 hours, and 5 hours) in order to determine the optimal timing for harvesting tissue. The preliminary study results showed that mice treated with high concentrations of ethanol died even before they reached several of the specific time points, which lead us to harvest tissues after 1 hour of ethanol administration, as all animals survived at least that long regardless of the ethanol dose.
2. Pathological Analysis of Damage to the Small Intestine Induced by Ethanol Administration
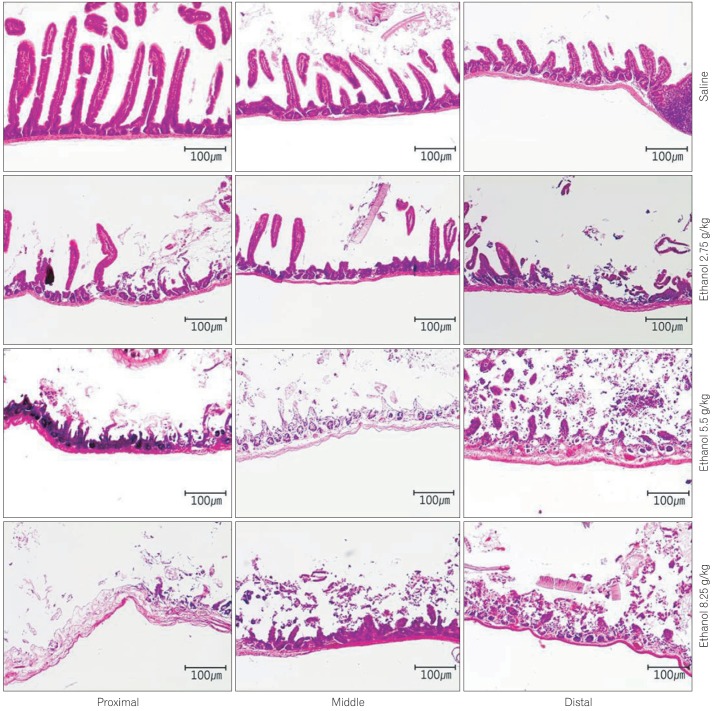
Pathological analyses were conducted to explore differences among the regions of the small intestine according to the concentration of ethanol administered. Regarding damage on mucosal membranes, samples from PBS-treated mice displayed well maintained villi and normal lymphoid follicles without infiltration of inflammatory cells. In contrast, mice treated with 2.75 g/kg of ethanol showed partial falling of the villi, slight edema of the glandular layers, damage to the villi in the proximal segments and more pronounced damage to the villi in distal segments. In mice treated with 5.5 g/kg, falling of the villi was observed more frequently, as were destroyed glandular layers, erosions, and ulcers. In particular, severe erosions and ulcers were detected in distal segments where lymphoid follicles were penetrated by inflammatory cells. Mice treated with 8.25 g/kg of ethanol showed an overall loss of glandular layers, extremely severe ulcers, and damage to the serosa. Furthermore, irregular expansion of the lumen was observed, possibly due to ischemic changes in the gastrointestinal tract (Fig. 1). Collectively, all ethanol-treated groups displayed more serious damage in distal segments as compared to proximal segments based on histological observations of the small intestines.
Fig. 1.
Pathology of the small intestinal mucosa and muscle induced by treatment with saline or ethanol (2.75 g/kg, 5.5 g/kg, 8.25 g/kg) (H&E, ×100). In saline-treated mice, villi were intact and normal lymphoid follicles were observed without infiltration of inflammatory cells. In mice treated with 2.75 g/kg of ethanol, focal falling off of villi and an edematous glandular layer were observed. These changes were more severe in the distal part with villi damage. In the group treated with 5.5 g/kg of ethanol, mulfifocal villi elimination, glandular layer destruction, erosions, and ulcers were observed with a severe aspect in the distal part. In mice treated with 8.25 g/kg of ethanol, diffuse damage extended to serosa, severe ulcers, and irregular expansion of lumen due to ischemic changes.
3. Changes in HSPs
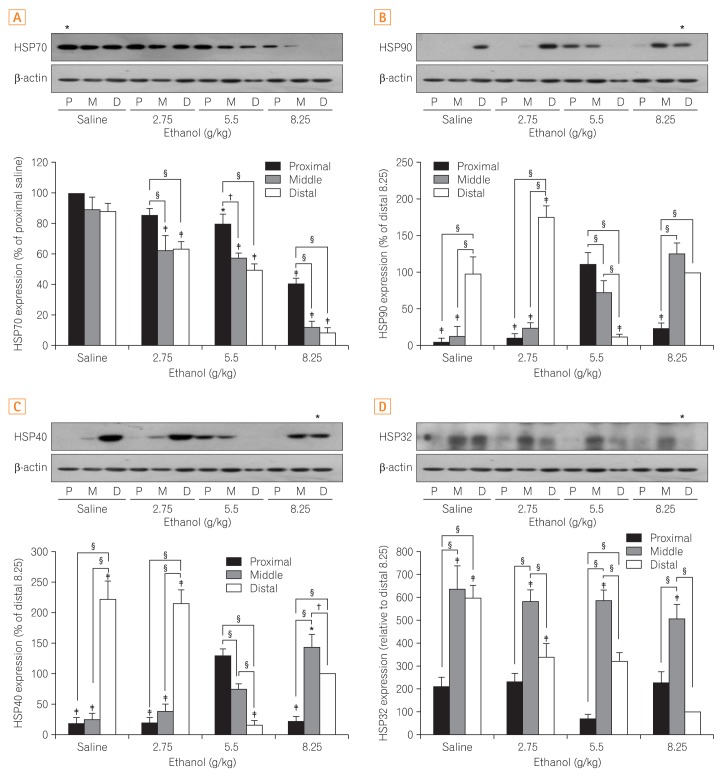
A variety of changes in the expression of HSPs were found in the small intestines in response to ethanol administration. Specifically, expression of HSP70 decreased in a dose-dependent manner. Such effects were more pronounced in the distal segments of the small intestines (Fig. 2A).
Fig. 2.
Comparison of the differences in expression of heat shock proteins (HSPs) in the small intestine. (A) HSP70, (B) HSP90, (C) HSP40, and (D) HSP32. The upper part of each figure shows the representative bands indicative of changes in the expression of HSPs by western blot analysis and the lower part shows a quantitatively obtained graph from the western blot analysis that was repeated 3 times. All results were obtained with 3 independent experiments and values are displayed as mean±SD. * and † indicate P<0.05. ‡ and § indicate P<0.01. P, proximal small intestine; M, middle small intestine; D, distal small intestine.
HSP40 was significantly increased in the control and low dose (2.75 g/kg) ethanol groups from the distal segment as compared to the proximal and middle segments. In contrast, HSP40 was significantly decreased in the distal segments of the small intestines from the 5.5 g/kg and 8.25 g/kg ethanol-treated groups (Fig. 2B).
Similar to HSP40, HSP90 was significantly elevated in the control group and decreased as the ethanol dosage increased in the distal segment of the small intestines (Fig. 2C). In addition, HSP90 was significantly higher in the proximal and middle segments of the small intestines of mice exposed to ethanol at 5.5 g/kg and was elevated in the middle segment in mice treated with 8.25 g/kg of ethanol, indicating site-specific differences and significant changes according to the concentration of ethanol are not observed in HSP90 expression.
In the control group, HSP32 was significantly increased in the middle and distal segments of the small intestines but decreased in the proximal segment by comparison. Although HSP32 was decreased in the distal segment in a dose dependent manner, its expression pattern was irregular in the proximal segment (Fig. 2D).
4. Changes in an Anti-oxidative Enzyme and Cytokines
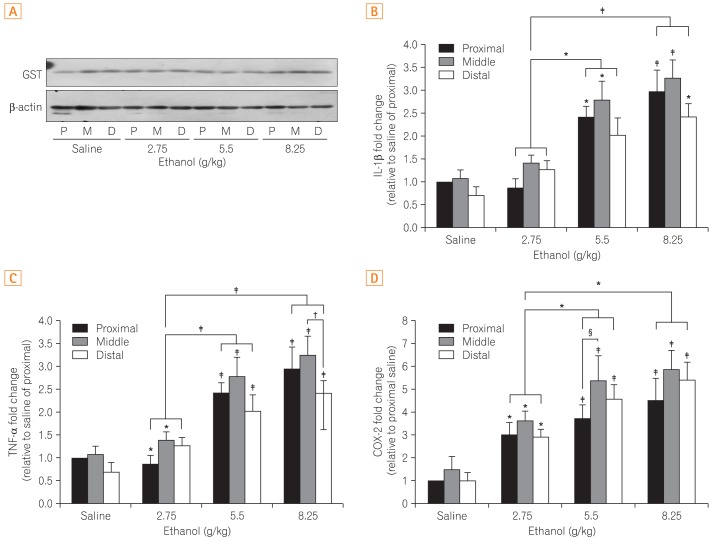
No difference in GST, an anti-oxidative enzyme, was noted between the control and ethanol-treated groups. Additionally, no changes were found in the different segments of the small intestines (Fig. 3A). Although expression of the cytokine TNF-α increased in a dose dependent manner, there was no difference among segments of the small intestines (Fig. 3B). Similarly, IL-1β expression increased with increasing ethanol dosages but did not demonstrated site-specific differences (Fig. 3C). The inflammatory mediator COX-2 was elevated without site-specific changes as well (Fig. 3D).
Fig. 3.
Comparison of the differences in the protein expression of (A) glutathione-S-transferase (GST) and (B) mRNA expression of interleukin-1β (IL-1β), (C) tumor necrosis factor-α (TNF-α), and (D) cyclooxygenase-2 (COX-2). Figure 3A shows the representative band indicative of changes in the expression of GST by western blot analysis. Figure 3B, C, and D show results obtained by extracting mRNA via reverse transcription-PCR assay to measure the expression of each gene. All results were obtained with 3 independent experiments and values are displayed as mean±SD. * and † indicate P<0.05. ‡ and § indicate P<0.01. P, proximal small intestine; M, middle small intestine; D, distal small intestine.
DISCUSSION
Acute exposure to ethanol is known to cause damage to the epithelial cells of the intestinal mucosa. Possible mechanisms underlying this phenomenon include increased intestinal permeability and the translocation of endotoxins by bacterial flora. As there are regional differences in intestinal bacteria, it is likely that structural and functional changes in the small intestines may differ from one to another region. In the current study, small intestinal damage was induced via acute administration of ethanol in order to compare various biochemical changes in the different regions and elucidate putative mechanisms underlying the damage.
Ethanol-induced damage to the small intestines has been associated with oxidative stress, which results in increased carbonylation and nitrotyrosination of cellular proteins including actin and the microtubule cytoskeleton. These changes are known to increase membrane permeability and induce the loss of integrity of tight junctions.3 Similar results were also demonstrated in a recent study that investigated ethanol-induced permeability of the epithelial cells of the lungs.8 Collectively, the results of the aforementioned studies suggest that oxidative stress may play a critical role in modulating intestinal permeability.
HSPs, molecular chaperones, are critical to cell survival and detrimental to protect cells against the harmful effects of stress-related stimuli. HSPs are synthesized in various cells when exposed not only to ethanol, but also to other environmental stressors. Although most HSPs mediate similar protective effects in response to stress, differences are apparent in their expression patterns and the biological roles depending on their class. For instance, HSP70 is known to be strongly induced by stress, whereas HSP90 is essentially expressed regardless of stress.9 An additional study reported that HSP70 was increased in non-steroidal anti-inflammatory drug-induced damage, while geranylgeranylacetone, a known HSP inducer used clinically as anti-ulcer drug, increased HSP70 in tissues of the small intestine and had a protective effect against damage to the intestinal mucosa.5 Furthermore, a protective effect of HSP70 has been demonstrated in intestinal inflammation, generating interest in potential novel roles of HSPs in the treatment of intestinal diseases.10 In rats, HSP70 and HSP32 were induced when ischemia-reperfusion injuries were generated.11 However, the roles of HSPs in the small intestine have not yet been fully elucidated. Therefore, the current study was conducted with the aim of investigating differences in the expression of HSPs in the small intestine and their potential roles in ethanol-induced intestinal damage. There are multiple HSPs with varying functions. In the current study, which was intended to examine the effects of HSPs on the different regions of the small intestine (i.e., proximal, middle, and distal parts) damaged by ethanol, a review of relevant literature led to the investigation of HSP70, HSP90, HSP40, and HSP32.
The results of the current study demonstrated distinct differences in damage to the small intestines of ICR mice depending on the concentration of ethanol and that damage was more pronounced in the distal region as compared to the rest of small intestines. When PBS was administered to controls, HSP40 and HSP90 were higher in the distal region while HSP70 was elevated regardless of the region examined. Conversely, HSP32 was lower in the proximal region in controls. In the ethanol-treated mice, it was difficult to find any specific correlation or site-specific associations between ethanol exposure and expression of HSPs, although differences in the expression levels of HSPs were detected and dependent on the region examined. HSP expression was significantly decreased in the distal small intestine after exposure to ethanol. HSP70 decreased in a dose-dependent manner in response to ethanol, particularly in the distal region. Similar findings were observed for HSP32, HSP40 and HSP90, however, displayed a site-specific pattern similar to that of HSP70 at a dose of 5.5 g/kg only, with different expression patterns found in the 2.75 g/kg and 8.5 g/kg ethanol-treated groups. When comparing the site-specific expression patterns of HSPs in this model, varying expression patterns and degrees of histological damage were found, in keeping with the known associations between the expression of HSPs and degrees of histological damage. In the small intestine of ICR mice, HSP70 generally showed the highest levels of expression and these levels were inversely correlated with the concentration of ethanol. It is important to note that the drastic change in HSP70 observed in distal regions was associated with severe tissue damage. In addition, the expression pattern of HSP32 was very similar to that of HSP70, suggesting that both HSPs may be critical in damage to the small intestine. However, it is difficult to elucidate the exact roles of these HSPs based on the results of the current study.
Upon the administration of ethanol, cytokines (i.e., TNF-α, and IL-1β) and inflammatory mediator (i.e., COX-2) were not showing any noticeable change among different regions of the small intestine, as found in macroscopic observations. Furthermore, no differences in the expression of GST, an anti-oxidative enzyme known to play an important role in protecting cells against oxidative stress, were detected between the control and ethanol groups, or among the regions of the small intestines in response to intestinal damage. However, it is difficult to exclude the possibility of an association between GST and intestinal damage, as the potential for differences in enzyme activity may be more significant than differences in protein expression levels. Additional studies examining putative associations between GST and intestinal damage may be warranted.
In the current study, varying magnitudes of histological damage and HSP expression in different regions of the small intestines were observed. One can expect that such site-specific changes are because we injected ethanol on lower right abdominal side. We decided to inject ethanol on the lower right side of the abdomen where there was a minimal risk of tissue damages due to a less dense distribution of blood vessels. However, the injection site itself may have influenced the results (i.e., a site specific effect of ethanol). This would seem unlikely, as HSP40 and HSP90 were exclusively increased in distal regions and HSP70 expression was elevated in a non-site-specific manner in the control group. Furthermore, HSP32 and HSP70 demonstrated similar expression patterns. Therefore, different patterns of HSP expression were likely due to their inherent features rather than the injection site. In order to provide further clarification regarding the effects of ethanol exposure on HSP expression patterns in the small intestines, a direct comparison should be made between different injection sites in future studies.
It may be possible for intestinal bacteria to impact patterns of HSP expression given the differences in distribution and classes of bacteria among regions of the small intestines. Physiological and environmental stress (e.g., microbial infection) may induce the synthesis of cellular protective HSPs on the intestinal epithelial cells. According to the results of previous studies, HSPs were modulated in response to multiple stimuli on the intestinal epithelial cells and such modulations were contingent on the exposure to intestinal probiotic bacteria.12 Therefore, the regional differences in HSP expression in the small intestine are likely attributable, at least in part, to the intestinal bacteria that differ in type and distribution throughout the small intestine. As opposed to the proximal region of the small intestine which contains few bacteria, more bacteria and their metabolites are present in the distal region and may result in more pronounced effects on HSP expression. Some HSPs are known to be expressed under stress-free environments to maintain cellular integrity. In a previous study that examined metronidazole administration in a small intestinal model, an intestinal bacterial flora demonstrated the ability to modulate physiological expression of the cellular protective HSPs 25 and 72.13 Although not examined in the current study, the administration of antibiotics may be useful in further elucidating associations between HSPs and intestinal bacteria in a mouse model.
For two different reasons, ethanol was administrated via intraperitoneal injection instead of oral exposure in the current study. First, the original goal of the study was to investigate differences among the regions of the small intestines in response to ethanol exposure. Although oral administration is the route of exposure for ethanol in humans, it may result in exposure to higher concentrations of ethanol in the proximal region compared to the distal regions and thereby make it difficult to analyze effects on the respective regions in a quantitative manner. Second, the bioavailability of ethanol in the small intestines differs due to varying degrees of gastric emptying. As such, exposure to ethanol via oral administration might be more suitable to examine chronic effects but is not recommended to determine acute effects. Therefore, ethanol was administered via intraperitoneal injection primarily to provide uniform concentrations over the small intestines in order to examine its impact on different regions. In previous studies, ethanol has been administered through intraperitoneal injection to achieve similar research objectives.14
The current study had another limitation. More extensive and accurate results may have been generated if the time course of the damaging effects of ethanol on the small intestines at the concentrations of ethanol high enough to make such changes (i.e., 5.5 g/kg or higher) was monitored. However, the initial study objective was to determine damage to varying regions of the small intestines. To better understand the mechanisms underlying ethanol-induced damage, further studies regarding the time course of effects as well as the magnitudes of intestinal damage dependent on exposure time should be conducted.
Collectively, results from the current study demonstrated that the expression patterns of the HSPs assessed varied in general and also in a region-specific manner. Specifically, more pronounced histological damage was found in the distal region and HSP70 and HSP32 were decreased in particular when ethanol was administered intraperitoneally. The endotoxins produced by bacterial flora may have influenced the expression of HSPs in ethanol-induced damage to the small intestine and future studies should address this possibility.
Footnotes
Financial support: This study was supported by the research grant from Kangwon National University School of Medicine 2011.
Conflict of interest: None.
References
- 1.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 2.Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374–3382. doi: 10.1111/j.1572-0241.2000.03347.x. [DOI] [PubMed] [Google Scholar]
- 3.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- 4.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano T, Tanaka K, Yamakawa N, et al. HSP70 confers protection against indomethacin-induced lesions of the small intestine. J Pharmacol Exp Ther. 2009;330:458–467. doi: 10.1124/jpet.109.152181. [DOI] [PubMed] [Google Scholar]
- 6.O'Mahony S, Shanahan F. Enteric microbiota and small intestinal bacterial overgrowth. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. Volume 2. 9th ed. Philadelphia: Elsevier Saunders; 2010. pp. 1769–1779. [Google Scholar]
- 7.Kim JA, Kim DK, Kang OH, et al. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol. 2005;5:209–217. doi: 10.1016/j.intimp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ, Hahm KB. Role of heat shock proteins in gastric inflammation and ulcer healing. J Physiol Pharmacol. 2009;60(Suppl 7):5–17. [PubMed] [Google Scholar]
- 10.Otaka M, Odashima M, Watanabe S. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem Biophys Res Commun. 2006;348:1–5. doi: 10.1016/j.bbrc.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto N, Kokura S, Okuda T, et al. Heme oxygenase-1 (Hsp32) is involved in the protection of small intestine by whole body mild hyperthermia from ischemia/reperfusion injury in rat. Int J Hyperthermia. 2005;21:603–614. doi: 10.1080/02656730500188599. [DOI] [PubMed] [Google Scholar]
- 12.Koninkx JF, Malago JJ. The protective potency of probiotic bacteria and their microbial products against enteric infections-review. Folia Microbiol (Praha) 2008;53:189–194. doi: 10.1007/s12223-008-0023-0. [DOI] [PubMed] [Google Scholar]
- 13.Arvans DL, Vavricka SR, Ren H, et al. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 2005;288:G696–G704. doi: 10.1152/ajpgi.00206.2004. [DOI] [PubMed] [Google Scholar]
- 14.Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30:1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]