Abstract
Failure to maintain protein homeostasis is associated with aggregation and cell death, and underies a growing list of pathologies including neurodegenerative diseases, aging and cancer. Misfolded proteins can be toxic and interfere with normal cellular functions, particularly during proteotoxic stress. Accordingly, molecular chaperones, the ubiquitin-proteasome system and autophagy together promote refolding or clearance of misfolded proteins. Here we discuss emerging evidence that the pathways of protein quality control (PQC) are intimately linked to cell architecture, and sequester proteins into spatially and functionally distinct PQC compartments. This sequestration serves a number of functions, including enhancing the efficiency of quality control; clearing the cellular milieu of potentially toxic species and facilitating asymmetric inheritance of damaged proteins to promote rejuvenation of daughter cells.
Introduction
Maintaining protein homeostasis (proteostasis) is essential for a functional cellular proteome [1,2]. Many diseases, including Alzheimer's, Parkinson's and Huntington's disease, are linked to accumulation of toxic misfolded proteins in aggregates and inclusions [1,2]. In order to maintain a properly functioning proteome, the cell has an extensive cellular protein quality control (PQC) network, involving chaperones and degradative pathways, that together ensure that proteins fold correctly and if misfolded, damaged or stress-denatured, that they are cleared from the cell [3–5]. Molecular chaperones maintain the solubility of misfolded species promoting their refolding or their degradation through the ubiquitin-proteasome system (UPS) and autophagy [6–9]. Sequestration of misfolded or aggregated proteins into inclusions was initially thought to be a second line of defense when quality control failed, for instance in response to proteasomal blockade [8]. This view deserves revisiting due to recent evidence that spatial sequestration of misfolded proteins is an early and physiological feature of cellular quality control, even in healthy cells [10]. Several distinct PQC compartments that concentrate different types of misfolded species are integral to cellular protein homeostasis (Table 1 and partially reviewed in [11]). We here review the function and components involved in the formation and clearance of these PQC compartments, and their relevance to understand diseases associated with protein aggregation.
Table 1.
Protein Quality Control Compartments in Eukaryotic Cells
| PQC compartment |
Conditions of appearance | Substrates | Cellular localization |
Sorting factors/ associated proteins |
Solubility | Cytoskeleton dependency |
References |
|---|---|---|---|---|---|---|---|
| Q-body | mild-severe heat stress; no proteasome inhibition | soluble, misfolded proteins | cortical ER | Hsp42, Hsp104, Hsp70, Hsp90, Hsp110, Ydj1/Hlj1 | soluble | no | [10] |
| JUNQ | severe heat stress; proteasome inhibition | soluble, ubiquitylated, misfolded proteins | juxtanuclear, perinuclear ER | Sis1, Btn2, Hsp70 proteasome | soluble | yes, actin | [14–15,21] |
| IPOD | normal growth conditions; no proteasome inhibition | amyloid proteins | perivacuolar | Hsp42, Btn2 (not for prions) | insoluble | debated, actin | [14–15,21] |
| Aggresome | proteasome inhibition | ubiquitylated, misfolded proteins | MTOC/SPB | Hsc70, Hsp40; HDAC6, parkin, ataxin-3, ubiquilin-1 | Insoluble (& soluble?) | yes, microtubule | [19] |
| ALIS | wide range of stress conditions | ubiquitylated, misfolded proteins | peripheral and juxtanuclear | ubiquitin; Atg8 | soluble | yes, microtubule and actin | [22] |
| ERAC/ERQC | genetic mutations that induce misfolding | misfolded membrane proteins/ERAD substrates | ER | Kar2p | soluble | no | [30] |
Misfolded protein species are sorted into distinct Protein Quality Control compartments
Intracellular and extracellular inclusions containing insoluble aggregates were originally observed in brains of patients affected with late-onset neurodegenerative diseases [12]. These diseases are associated with proteins that have a high propensity to aggregate in vitro and in vivo into highly ordered beta-sheet rich aggregates called amyloids [2,13]. Under conditions of proteasome inhibition and stress, inclusions containing non-amyloid amorphous aggregates, as well as inclusions containing detergent-soluble misfolded proteins, have been also described in yeast and mammalian cells [14–20]. It is now clear that these various inclusions represent distinct Protein Quality Control compartments formed through defined pathways in order to sequester misfolded species from the cellular milieu and to facilitate their management by the protein homeostasis machineries. While better characterized in yeast, these PQC pathways and compartments appear largely conserved across eukaryotic cells [14,15,19,21–23], using analogous chaperones and sorting factors.
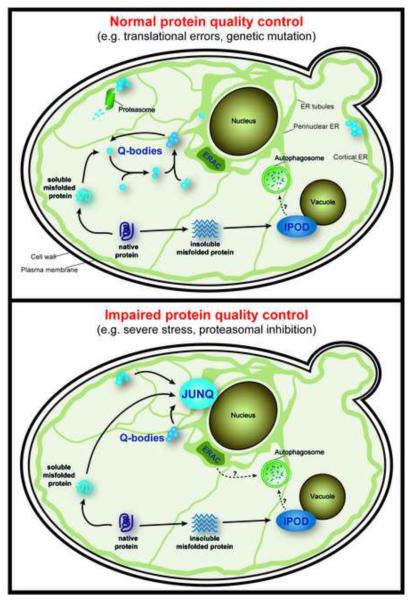
Upon impairment of proteasomal function, destabilized and misfolded proteins which normally are degraded in a chaperone- and UPS-dependent manner, are sequestered into the perinuclear endoplasmic reticulum (ER)-associated JUNQ (JUxtaNuclear Quality control) compartment (Figure 1) [14,15,21]. In contrast, insoluble aggregates of amyloid-forming proteins, such as prions or polyglutamine expanded Huntingtin (Htt), are sequestered in the IPOD (Insoluble PrOtein Deposit) compartment that resides near the vacuole (Figure 1) [14,20]. Non-amyloidogenic QC substrates, i.e. missense mutations and stress-denatured proteins, can also be targeted to the IPOD under severe stress or when their ubiquitination is blocked [14]. Under prolonged exposure to extreme cellular stress, soluble proteins can also localize to another, less structured, peripheral cytoplasmic compartment that appears to be long-lived and may be a site of permanent sequestration of toxic soluble proteins [15].
Figure 1. The spatial distribution of the different PQC compartments within a yeast cell.
Model illustrating the different PQC compartments and their subcellular locations in yeast grown under normal growth conditions (top) or conditions of impaired protein quality control (bottom). Similar compartments have been described in mammalian cells, including the aggresome and ALIS (see text for details).
A recent study showed that misfolded protein sequestration also occurs in the absence of proteasome impairment [10]. Misfolded proteins are spatially sequestered into dynamic, ER-anchored, structures called Q-bodies, even as they are degraded by the UPS [10]. Sequestration into Q-bodies is chaperone-dependent and likely serves to clear the cytoplasm from toxic soluble, misfolded species. Indeed, impairing Q-body formation reduces cellular fitness to stress. Q-bodies continuously coalesce dynamically into larger puncta, suggesting that to formation of the JUNQ could be an endpoint of this pathway when clearance is impaired by proteasomal inhibition.
In mammalian cells, misfolded proteins also partition into distinct inclusions depending on their solubility and properties [14,24]. While similar to yeast, mammalian PQC may rely on additional factors and be subject to more complex regulation stemming from their more elaborate cell architecture. Insoluble aggregated proteins are concentrated into an aggresome, which is located at the centrosome [19]. Misfolded proteins are transported to the aggresome via binding to the histone deacetylase 6 and dynein proteins on the microtubules [25]. While probably analogous to the IPOD, the aggresome is surrounded by a vimentin cage [19], absent in yeast. Aggresomes are associated with a many PQC proteins including chaperones and components of the UPS and autophagy, and have been proposed to be involved in protein refolding, clearance, terminal sequestration, and aggregate retention [19,26,27].
An example of how mammalian cells elaborate on spatial PQC pathways is the ALIS (Aggresome-Like Induced Structures), which concentrates polyubiquitinated proteins for subsequent proteasomal degradation and MHC class I-dependent antigen presentation (Figure 1) [22]. Peripheral and perinuclear-localized ALIS form in the cytosol of many cell types as a response to inflammation, infection or ER stress, in professional antigen-presenting dendritic cellsthey are called DALIS [22,28–30]. Autophagic proteins like p62 co-aggregate with the ALIS substrates [30]. While ALIS do not accumulate proteasomes like other PQC compartments, proteasome activity is required for clearance [28,29].
Quality control in the ER also involves spatial sequestration. One of the first described PQC compartments is the ER-derived Quality control Compartment (ERQC) residing near the centriole and concentrating soluble misfolded proteins for ER-Associated Degradation (ERAD) by the UPS [31]. Similarly, yeast form a membrane-bound ER-Associated Compartment (ERAC), which serves as a holding site before ERAD (Figure 1) [32]. Aggregated proteins are also cleared from the ER by the autophagy-lysosome pathway [33]. Thus, both cytoplasmic and ER-associated quality control direct misfolded proteins to spatially distinct PQC compartments.
Functional consequences of spatial quality control
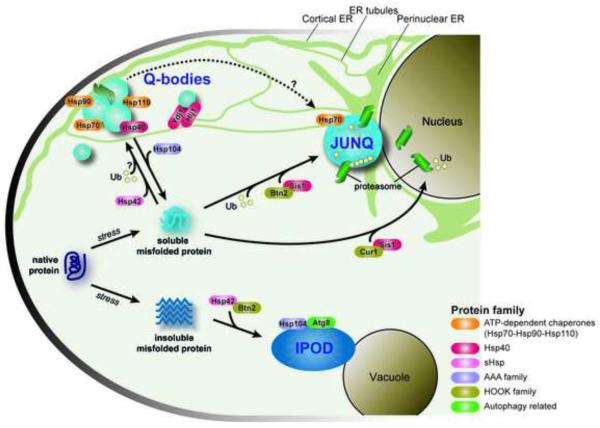
What is the function of spatially sequestering misfolded proteins in such a diverse range of compartments? Each compartment seems to be tied to a distinct fate for the misfolded protein, including refolding by a specific set of chaperones, clearance by the UPS or autophagy, or terminal sequestration from the cellular milieu. One function of PQC compartments is enhancing the efficiency of protein quality control by restricting the diffusion of misfolded proteins and concentrating them together with chaperones and clearance factors. Another important function is to protect the cellular milieu from potentially toxic misfolded species that can sequester chaperones and interfere with folding of sensitive nascent polypeptides and assembly of essential complexes and structures [17,34]. The JUNQ and Q-bodies concentrate proteins in a detergent-soluble state with chaperones and 26S proteasomes, likely acting in triage and storage of misfolded proteins keeping them competent for both refolding and degradation. Terminal sequestration of proteins in the IPOD and aggresome is thought to protect the cell from toxic misfolded species [14], as highlighted by studies showing that neurons are more likely to die if they fail to sequester aggregation-prone Htt into a large insoluble inclusion [16,35]. However, the IPOD is not entirely static as it also contains the potent ATP-dependent disaggregase Hsp104 and the autophagy-related protein Atg8 which may help clear aggregates (Figure 2) [14,15].
Figure 2. Pathways of spatial processing of misfolded proteins within the cell.
Stress-denatured proteins interact with a number of sorting factors directing the misfolded proteins to individual PQC compartments within the cell, leading to refolding, degradation or terminal sequestration of the potentially toxic species.
Misfolded protein sequestration is also thought to rejuvenate daughter cells, clearing them of cellular damage during asymmetric cell division. In yeast, both the JUNQ and the IPOD are retained by the mother cell [14,36,37]. Clearing daughter cells from potentially harmful material may extend their replicative lifespan while the mother cell ages, losing the capacity to divide. The cytoskeleton, together with the dynamic network of cellular membranes may mediate segregation of PQC compartments between mother and daughter [38] [39]. either through actively transport along the actin cytoskeleton from the bud back to the mother cell [38] or randomly by confined diffusion retaining inclusions in the mother cell [39].
In mammalian cells, asymmetric inheritance of PQC compartments also occurs during stem cell division [37,40,41]. Stem cells face a unique protein homeostasis challenge as they must maintain the potential to regenerate organs and tissues throughout the life of an organism, even under conditions of stress. Notably, in some cases the dividing stem cells is cleared of damaged proteins, in others, the inclusion stays in the stem cells to protect the differentiated daughter cell [41]. This paradoxical finding was linked to the longevity of either cell, with the longest living cell remaining cleared of damage. These results highlight the complexity of control of PQC compartment inheritance in mammalian cells [37,40]. Future studies should clarify the processes and organelles regulating asymmetric aggregate segregation, and how this process affects cell fate and fitness.
Molecular and cellular determinants of spatial Protein Quality Control
Formation of PQC compartments is an active process involving chaperones, ubiquitination, as well as sorting factors interacting with cellular structures, including the nuclear membrane, the ER network and the cytoskeleton. ATP-dependent molecular chaperones of the Hsp70, Hsp90 and Hsp110 families, previously shown to be important for refolding and degradation of misfolded or stress-denatured proteins, are very important for the formation of Q-bodies and their maturation into the JUNQ (Figure 2) [10,14]. Less is known about the formation of the IPOD, but it is dependent upon specific chaperone activities [14]. Sorting to different PQC compartments has distinct chaperone requirements, raising the question of what determines if a given misfolded protein is selected for sequestration into a specific PQC compartment. The activity and specificity of Hsp70s and Hsp90s is regulated by many co-chaperones, and these may play key roles in the selection and targeting of misfolded substrates to specific PQC compartments. This was shown for J-domain proteins (often called Hsp40s), which regulate Hsp70 ATP hydrolysis and substrate binding [42]. Two ER-associated Hsp40 co-chaperones, Ydj1 and Hlj1 [43,44], contribute redundantly to Q-body formation and maturation ([10] and Figure 2), perhaps linking these cytoplasmic structures to the ER membrane. The Hsp40 Sis1 (DnaJB1 in mammals) is reported to mediate the deposition and associated proteasomal degradation of misfolded cytosolic proteins in and around the nucleus [21,45] (Figure 2). As a consequence of cellular stress and insufficient Sis1 shuttling capacity, inclusions may form, not only in the nucleus but also at peripheral and juxtanuclear sites [21,45], suggesting a potential role for Sis1 in Q-body maturation into the JUNQ.
The ATP-independent small heat shock proteins (sHSPs) play an important role in spatial quality control. sHSPs form large oligomeric complexes with misfolded proteins to maintain them in a more soluble state. The physiological state of the cell may direct the function and spatial sorting capacity of Hsp42. Without proteasome inhibition, the sHSP Hsp42 drives the formation of Q-bodies, both in the juxtanuclear region as well as in the cell periphery [10] (Figure 2). Upon proteasomal blockade, Hsp42 escorts its substrates to peripheral IPOD-like aggregates but does not associate with the JUNQ. Another yeast sHSP, Hsp26, is only activated under more extreme stress conditions and colocalizes with peripheral aggregates [15]. The disaggregase Hsp104 is essential for IPOD formation and Q-body maturation, and enhances cellular fitness during stress [10,14] (Figure 2). Interestingly, deletion of Hsp104 is beneficial in basal unstressed conditions, which may explain why mammalian cells, whose control of homeostasis is non-cell autonomous [18,46], have dispensed with this chaperone [10].
The ER membrane, which surrounds the nucleus and extends as a tubular network throughout the entire cell [47], is emerging as a central organizing framework for PQC [10,14]. Disruption of the reticular ER network impairs misfolded protein degradation and abrogates Q-body formation [10]. The cytoskeleton has also been proposed as a main determinant in the spatial trafficking to the various PQC sites. This function could be direct [38] or indirect, for instance due to its role in the dynamics of the peripheral ER [48,49]. An important consideration in interpreting studies on the role of the cytoskeleton is the recent observation that prolonged treatment with cytoskeleton depolymerizing agents, targeting either actin or tubulin, is in itself highly proteotoxic and leads to a proteostatic collapse that indirectly affect misfolded protein management in the cell [10]. Recently, the related HOOK family proteins Cur1 and Btn2 have been shown to promote differential sorting and deposition of misfolded proteins into cytosolic compartments by physically and functionally interacting with chaperones [21] (Figure 2). Association of the short lived, stress-induced Btn2 with Hsp42 drives the sorting of the non-amyloid substrates like VHL to the IPOD, but not for amyloid protein sorting to the IPOD [21]. On the other hand, Btn2 binding to Sis1 mediates the trafficking of soluble misfolded proteins to the JUNQ.
Implications of spatial PQC sequestration in disease pathogenesis and treatment
A better understanding of spatial quality control in healthy cells can help understand what goes wrong in diseases whose hallmark is the accumulation of protein aggregates, including Alzheimer's, Parkinson's, and Huntington's diseases, Amyotrophic Lateral Sclerosis (ALS) and many others. Genetic mutations or unknown environmental factors lead to misfolding of specific proteins that affect different neuronal types for each of these disorders. Misfolded protein inclusions are also linked to non-neuronal diseases including lysosomal storage disorders (LSD), cystic fibrosis, cancer and even aging [50]. Targeting of misfolded proteins to PQC compartments might be implicated in other types of diseases. For instance, in multiple LSDs, destabilizing mutations in ERAD substrates are common [51,52] and defects in PQC sorting factors have been implicated in disease pathogenesis [53]. Accumulation and sequestration of misfolded proteins may play a role in cancer, given recent findings that aneuploid yeast strains contain Hsp104-positive aggregates of endogenous proteins [54]. Further, mutations in the tumor suppressor p53 lead to a higher aggregation propensity which induces misfolding and coaggregation of normal p53 as well as its paralogs p63 and p73 into inclusions [55].
While the role of protein aggregates in disease pathogenesis is unclear, aggregation is a clear indicator of cellular dysfunction. The notion that quality control is spatially localized sheds new light on the relationship between inclusions and disease. Thus, the inclusion itself may not be the problem, but its appearance may reflect a problem in the balance between production and clearance of misfolded proteins. This is consistent with observations that forming inclusions containing misfolded proteins is protective in a number of settings [56]. It is possible that sequestering toxic, misfolded proteins confers protection early in disease; however, at later stages critical factors may become ensnared in inclusion bodies resulting in increased cellular dysfunction. Indeed, aggregates of misfolded proteins can sequester chaperones such as Sis1, Hsp110 and NAC, further impairing the process of quality control as well as translation and folding of new proteins [45,57]. This in turn results in the further aggregation of quality control substrates as well as newly made misfolding-sensitive precursors, for instance ribosome biogenesis intermediates [17,57]. Therefore, it is conceivable that distinct therapeutic strategies will be necessary at the different stages of disease progression. Interestingly, a large body of literature suggesting that quality control declines with aging [58–60], and in this regard, maintaining a functional spatial PQC machinery may be an important component of healthy aging, as many healthy aged brains contain protein aggregate in post-mortem analyses [61].
Conclusions and future directions
Growing evidence indicates that spatial sequestration of misfolded protein species is an early cellular response to protein misfolding and does not necessarily result from proteostatic collapse or disease. A complex picture is beginning to emerge linking cellular architecture determinants to the cellular degradation and folding systems to establish spatial quality control. Future studies will have to determine how the properties of a given misfolded protein and/or the state of a given cell lead to the generation of toxic rather than protective inclusions. A better understanding of this process may enable the development of new approaches to treat misfolding and aggregation diseases. Guiding misfolded proteins towards beneficial PQC compartments, or away from potentially detrimental ones may represent the next direction of therapeutic design in the field of protein misfolding disorders (reviewed in [59,62]). Thus, it remains critical to understand the cell biology involved in PQC body formation in order to design appropriate therapeutic approaches for the wide range of misfolding diseases.
Highlights.
Spatial sequestration of misfolded proteins is an early, normal response to stress.
PQC compartments spatially restrict misfolded proteins thus enhancing degradation.
The ER acts as a dynamic network for the spatial distribution of misfolded proteins.
Spatial protein sequestration promotes rejuvenation of daughter cells in mitosis.
Altered PQC is associated with neurodegenerative disorders, cancer and aging.
Acknowledgements
Work in the Frydman lab is supported by the NIH and a Senior Scholar Award from the Ellison Foundation to JF. WV is supported by the Marie Curie International Outgoing Fellowship Program (WV, FP7-PEOPLE-2011-IOF; contract no. [303096 FOLDEG]). ES is supported by a postdoctoral fellowship from NIH.
Abbreviations
- CFTR
cystic fibrosis transmembrane receptor
- ER
endoplasmic reticulum
- ERAC
ER-associated complex
- ERAD
ER-associated degradation
- Htt
Huntingtin
- IPOD
insoluble protein deposition
- JUNQ
juxtanuclear quality control compartment PM plasma membrane
- PQC
protein quality control
- SQC
spatial quality control
- UPR
unfolded protein response
- UPS
ubiquitin proteasome system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Vendruscolo M, Knowles TP, Dobson CM. Protein solubility and protein homeostasis: a generic view of protein misfolding disorders. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 4.Roth DM, Balch WE. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol. 2011;23:126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Kastle M, Grune T. Interactions of the proteasomal system with chaperones: protein triage and protein quality control. Prog Mol Biol Transl Sci. 2012;109:113–160. doi: 10.1016/B978-0-12-397863-9.00004-3. [DOI] [PubMed] [Google Scholar]
- 7.Houck SA, Singh S, Cyr DM. Cellular responses to misfolded proteins and protein aggregates. Methods Mol Biol. 2012;832:455–461. doi: 10.1007/978-1-61779-474-2_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Retzlaff M, Roos T, Frydman J. Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol. 2011;3:a004374. doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- 10.Escusa-Toret S, Vonk WI, Frydman J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol. 2013 doi: 10.1038/ncb2838. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This paper describes for the first time sorting of misfolded protein into transient PQC compartments under basal conditions, which the authors termed Q-bodies. These findings indicate that sequestration of misfolded proteins is an early event during protein quality control that enhances fitness during stress.
- 11.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012;151:1163–1167. doi: 10.1016/j.cell.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa Y, Nukina N. Functional diversity of protein fibrillar aggregates from physiology to RNA granules to neurodegenerative diseases. Biochim Biophys Acta. 2013;1832:1271–1278. doi: 10.1016/j.bbadis.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Specht S, Miller SB, Mogk A, Bukau B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J Cell Biol. 2011;195:617–629. doi: 10.1083/jcb.201106037. [DOI] [PMC free article] [PubMed] [Google Scholar]; *In this paper, the show that the small HSP Hsp42 directs the sorting of misfolded proteins to the peripheral PQC compartments under stress conditions.
- 16.Miller J, Arrasate M, Shaby BA, Mitra S, Masliah E, Finkbeiner S. Quantitative relationships between huntingtin levels, polyglutamine length, inclusion body formation, and neuronal death provide novel insight into huntington's disease molecular pathogenesis. J Neurosci. 2010;30:10541–10550. doi: 10.1523/JNEUROSCI.0146-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]; **A quantitative pulsed-SILAC proteomic analysis comparing the interactome of toxic and non-toxic beta-protein aggregates revealed that toxic aggregates contain chaperones and preexisting misfolded proteins but also sequester nascent polypeptides.
- 18.Gidalevitz T, Prahlad V, Morimoto RI. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyedmers J, Treusch S, Dong J, McCaffery JM, Bevis B, Lindquist S. Prion induction involves an ancient system for the sequestration of aggregated proteins and heritable changes in prion fragmentation. Proc Natl Acad Sci U S A. 2010;107:8633–8638. doi: 10.1073/pnas.1003895107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23:3041–3056. doi: 10.1091/mbc.E12-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]; **The data presented in this paper define a clear role for Sis1, Btn2 and Cur1 as sorting factors for misfolded proteins to the spatially distinct PQC compartments under stress conditions.
- 22.Szeto J, Kaniuk NA, Canadien V, Nisman R, Mizushima N, Yoshimori T, Bazett-Jones DP, Brumell JH. ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy. 2006;2:189–199. doi: 10.4161/auto.2731. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg SJ, Lyakhovetsky R, Werdiger AC, Gitler AD, Soen Y, Kaganovich D. Compartmentalization of superoxide dismutase 1 (SOD1G93A) aggregates determines their toxicity. Proc Natl Acad Sci U S A. 2012;109:15811–15816. doi: 10.1073/pnas.1205829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto G, Kim S, Morimoto RI. Huntingtin and mutant SOD1 form aggregate structures with distinct molecular properties in human cells. J Biol Chem. 2006;281:4477–4485. doi: 10.1074/jbc.M509201200. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 26.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 27.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 28.Canadien V, Tan T, Zilber R, Szeto J, Perrin AJ, Brumell JH. Cutting edge: microbial products elicit formation of dendritic cell aggresome-like induced structures in macrophages. J Immunol. 2005;174:2471–2475. doi: 10.4049/jimmunol.174.5.2471. [DOI] [PubMed] [Google Scholar]
- 29.Lelouard H, Gatti E, Cappello F, Gresser O, Camosseto V, Pierre P. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 2002;417:177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 30.Liu XD, Ko S, Xu Y, Fattah EA, Xiang Q, Jagannath C, Ishii T, Komatsu M, Eissa NT. Transient aggregation of ubiquitinated proteins is a cytosolic unfolded protein response to inflammation and endoplasmic reticulum stress. J Biol Chem. 2012;287:19687–19698. doi: 10.1074/jbc.M112.350934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell. 2001;12:1711–1723. doi: 10.1091/mbc.12.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huyer G, Longsworth GL, Mason DL, Mallampalli MP, McCaffery JM, Wright RL, Michaelis S. A striking quality control subcompartment in Saccharomyces cerevisiae: the endoplasmic reticulum-associated compartment. Mol Biol Cell. 2004;15:908–921. doi: 10.1091/mbc.E03-07-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida Y, Yamamoto A, Kitamura A, Lamande SR, Yoshimori T, Bateman JF, Kubota H, Nagata K. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009;20:2744–2754. doi: 10.1091/mbc.E08-11-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duttler S, Pechmann S, Frydman J. Principles of cotranslational ubiquitination and quality control at the ribosome. Mol Cell. 2013;50:379–393. doi: 10.1016/j.molcel.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 36.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 37.Ouellet J, Barral Y. Organelle segregation during mitosis: Lessons from asymmetrically dividing cells. J Cell Biol. 2012;196:305–313. doi: 10.1083/jcb.201102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Zhou C, Slaughter BD, Unruh JR, Eldakak A, Rubinstein B, Li R. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study reports that segregation of protein aggregates is not reliant on the cytoskeleton, but rather results from a random diffusion of protein aggregates between mother and daughter cells.
- 40.Prinsloo E, Setati MM, Longshaw VM, Blatch GL. Chaperoning stem cells: a role for heat shock proteins in the modulation of stem cell self-renewal and differentiation? Bioessays. 2009;31:370–377. doi: 10.1002/bies.200800158. [DOI] [PubMed] [Google Scholar]
- 41.Bufalino MR, DeVeale B, van der Kooy D. The asymmetric segregation of damaged proteins is stem cell-type dependent. J Cell Biol. 2013;201:523–530. doi: 10.1083/jcb.201207052. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study shown that during stem cell division, the damaged proteins are asymmetrically inherited by the cell with the shortest lifespan, preventing the accumulation of proteins damaged by reactive oxygen species. It is hypothesized that this segregation occurs via stem cell niche-dependent and –independent mechanisms.
- 42.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caplan AJ, Tsai J, Casey PJ, Douglas MG. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J Biol Chem. 1992;267:18890–18895. [PubMed] [Google Scholar]
- 44.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell. 2004;15:4787–4797. doi: 10.1091/mbc.E04-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SH, Kukushkin Y, Gupta R, Chen T, Konagai A, Hipp MS, Hayer-Hartl M, Hartl FU. PolyQ Proteins Interfere with Nuclear Degradation of Cytosolic Proteins by Sequestering the Sis1p Chaperone. Cell. 2013;154:134–145. doi: 10.1016/j.cell.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.English AR, Voeltz GK. Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol. 2013;5:a013227. doi: 10.1101/cshperspect.a013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman JR, Voeltz GK. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 2011;21:709–717. doi: 10.1016/j.tcb.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci U S A. 2010;107:6894–6899. doi: 10.1073/pnas.0911482107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alberti S. Molecular mechanisms of spatial protein quality control. Prion. 2012;6:437–442. doi: 10.4161/pri.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grace ME, Newman KM, Scheinker V, Berg-Fussman A, Grabowski GA. Analysis of human acid beta-glucosidase by site-directed mutagenesis and heterologous expression. J Biol Chem. 1994;269:2283–2291. [PubMed] [Google Scholar]
- 52.Jeyakumar M, Butters TD, Dwek RA, Platt FM. Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol Appl Neurobiol. 2002;28:343–357. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 53.Chattopadhyay S, Muzaffar NE, Sherman F, Pearce DA. The yeast model for batten disease: mutations in BTN1, BTN2, and HSP30 alter pH homeostasis. J Bacteriol. 2000;182:6418–6423. doi: 10.1128/jb.182.22.6418-6423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26:2696–2708. doi: 10.1101/gad.207407.112. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study demonstrates that aneuploidy, a hallmark of cancer, leads to an imbalance of protein homeostasis that results in formation of aggregates and impairment of quality control
- 55.Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, Cornelis A, Rozenski J, Zwolinska A, Marine JC, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe KJ, Cyr DM. Amyloid in neurodegenerative diseases: friend or foe? Semin Cell Dev Biol. 2011;22:476–481. doi: 10.1016/j.semcdb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirstein-Miles J, Scior A, Deuerling E, Morimoto RI. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 2013;32:1451–1468. doi: 10.1038/emboj.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calamini B, Morimoto RI. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr Top Med Chem. 2012;12:2623–2640. doi: 10.2174/1568026611212220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer's disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]