Figure 2.
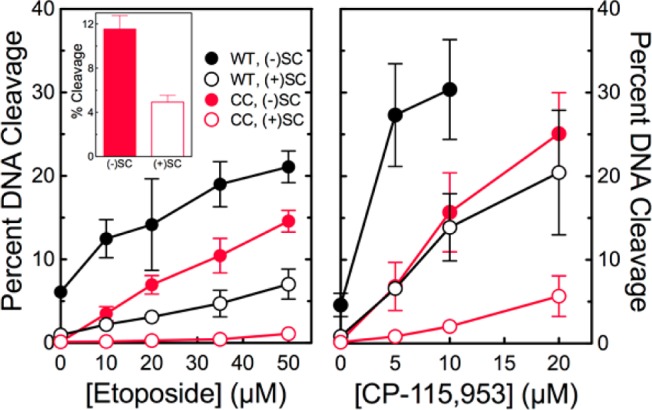
The catalytic core of human topoisomerase IIα preferentially cleaves negatively supercoiled DNA in the presence of etoposide and CP-115,953. The ability of wild-type topoisomerase IIα (WT, black) and the catalytic core (CC, red) to cleave negatively [(−)SC, filled circles] or positively [(+)SC, empty circles] supercoiled plasmid DNA in the presence of etoposide (left) or CP-115,953 (right) is shown. Results for CP-115,953 are not shown at concentrations above 10 μM with the wild-type enzyme because the drug induced multiple cleavage events per plasmid. The inset shows cleavage induced by the catalytic core in the presence of 250 μM etoposide. Error bars represent the standard deviation of at least three independent experiments.
