Figure 1.
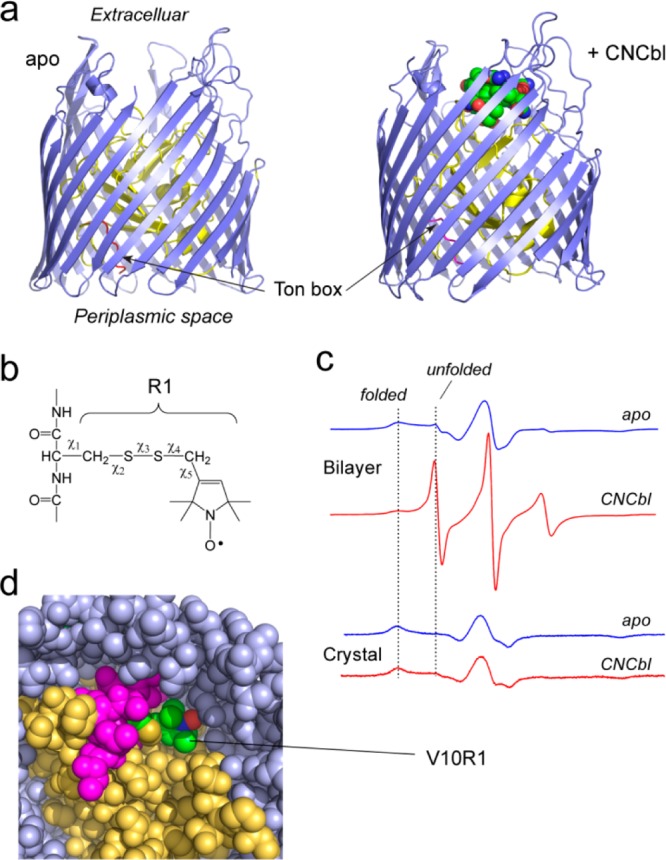
Substrate-dependent conformational changes in BtuB are not seen in the crystal environment. (a) Crystal structures of the Escherichia coli vitamin B12 transporter, BtuB, in the apo and ligand bound forms (PDB IDs 1NQE and 1NQH, respectively). BtuB and all TBDTs consist of a 22-standed β-barrel (blue) and an N-terminal core domain (yellow), which fills the barrel. A conserved motif called the Ton box (red) directly interacts with the inner membrane protein TonB. In both the apo and substrate bound crystal forms, the Ton box is folded within the interior of the protein. (b) The nitroxide side chain, R1, is formed by reaction of a methanethiosulfonate label with a reactive cysteine residue. This label is one of several different labels that have been developed for site-directed spin labeling.6 (c) EPR spectra from BtuB labeled at position 10 in the Ton box (BtuB/V10R1).28 The substrate induced change in the EPR spectrum is observed in bilayers but is not seen in the environment of the protein crystal. (d) Crystal structure of BtuB/V10R1 in the presence of substrate (PDB ID 3M8D).28 The label is in tertiary contact within the protein interior, consistent with the EPR spectra in panel c. All structures were rendered using PyMol (Schrödinger, Portland, OR). Panels c and d reproduced from ref (28). Copyright 2010 Biophysical Society.
