Figure 1.
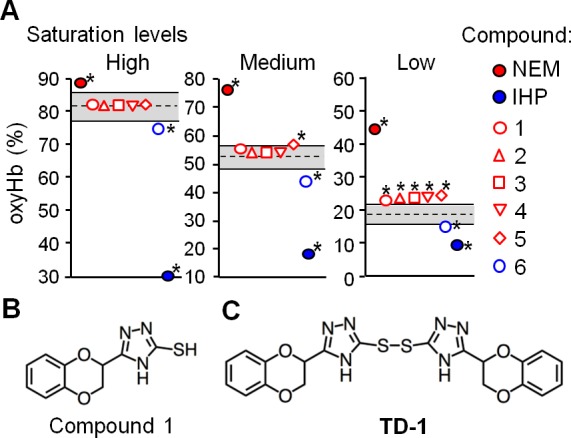
Identification of small molecules that alter the oxygen affinity of normal human hemoglobin. (A) The fraction of hemoglobin that binds oxygen (oxyHb%) was evaluated in the absence of added compounds (oxyHb%ctrl) or in their presence (oxyHb%cpd). The dashed line indicates the mean value of oxyHb%ctrl under each condition (high, medium, and low oxyHb saturation conditions) and the gray area bounded by solid lines indicates the range of the mean value ± 2.5 standard deviations of oxyHb%ctrl. Each symbol represents the mean value of oxyHb%cpd measured in quadruplicate. Incubation of hemoglobin with NEM and IHP increased and decreased the oxyHb%, respectively. Asterisks indicate compounds that the mean value of oxyHb%cpd was out of the gray area under each condition. (B) The chemical structure of compound 1. (C) The chemical structure of TD-1 (disulfide form of compound 1).
