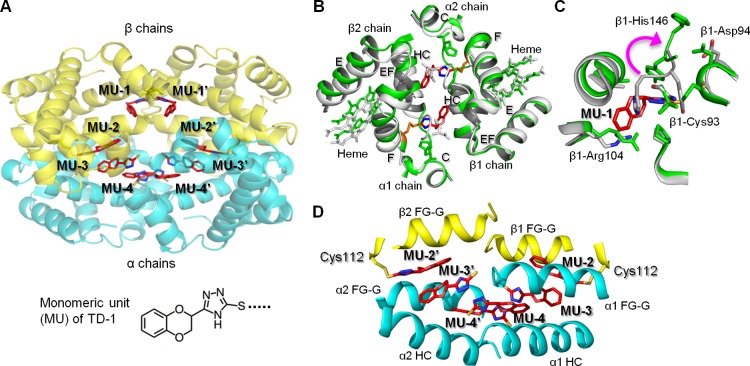
Figure 3.
The relaxed state (R3-state) of hemoglobin is stabilized by TD-1 via covalent and non-covalent bonding. (A) Structure of R3-state hemoglobin with TD-1 as monomeric units (MUs, red sticks). α-chains of hemoglobin are blue, and β-chains are yellow. Heme is not shown for clarity. (B) Binding of MU-1/MU-1′ (red sticks) to β-Cys93 (orange sticks) induces large tertiary and quaternary structural perturbations. Hemoglobin in the absence of MUs (R3 state, PDB code 1YZI) is shown in white/gray and hemoglobin in the presence of MUs (PDB code 4NI0) is shown in green. (C) Superposition of COHb without MUs (white ribbons) and COHb-MU complex (green ribbons). MU-1 is shown as red sticks. A magenta arrow represents the movement of β-His146 associated with MU-1/MU-1′ binding of Cys93. (D) Interactions of MU-2/MU-2′, MU-3/MU-3′, and MU-4/MU-4′ in the water cavity help to tie the four hemoglobin subunits together.