Figure 6. The Pellino2 wing extends the substrate-binding surface.
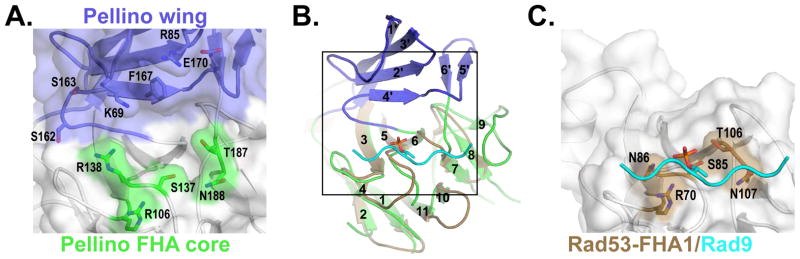
The Pellino2 FHA-like domain and the Rad53 FHA1/Rad9 complex are shown looking onto the phosphothreonine binding site, a 180° rotation about a vertical axis with respect to Fig. 5.
(A) The apposition of the Pellino2 wing and FHA core result in a binding pocket that is lined with hydrophobic amino acids and rimmed on one side by amino acids (green) implicated in direct contact to the phosphothreonine, and on the other side by charged and polar side chains from the wing loops (blue).
(B) The Rad53-FHA1 (beige) is show superimposed on the Pellino FHA core (green). The phosphopeptide bound to Rad53-FHA1 is shown in cyan. The box indicates the area of detail shown in A and C.
(C) The phosphopeptide binds to a groove on the surface of Rad53-FHA1 analogous to the lower rim of the pocket highlighted in A.
