Abstract
In higher primates development of the adult population of Leydig cells has received little attention. Here, the emergence of 3β-hydroxysteroid dehydrogenase (HSD3B) positive cells in the testis of the rhesus monkey was examined during spontaneous puberty, and correlated with S-phase labeling in the interstitium at this critical stage of development. In addition, the relative role of LH and FSH in initiating the pubertal expansion of Leydig cells was studied by precociously stimulating the juvenile testis in vivo with pulsatile 11-day infusions of recombinant LH and FSH, either alone or in combination. At the time of castration, testes were immersion fixed in Bouin’s, embedded in paraffin and sectioned at 5 μm. Leydig cells/testis were enumerated using HSD3B as a Leydig cell marker. Leydig cell number per testis increased progressively during puberty to reach values in the adult approximately 10 fold greater than in early pubertal animals. The rise in cell number was associated with an increase in nuclear diameter. That the pubertal expansion of Leydig cell number was driven primarily by the increase in LH secretion at this stage of development was suggested by the finding that precocious stimulation of mid juvenile monkeys with LH, either alone or in combination with that of FSH, resulted in a 20 to 30 fold increase in the number of HSD3B positive cells. Interestingly, precocious FSH stimulation, alone, also resulted in appearance of Leydig cells as indicated by the occasional HSD3B positive cell in the interstitium. The nuclear diameter of these Leydig cells, however was less than that of those generated in response to LH.
Keywords: Rhesus monkey, Leydig cell, LH, FSH, testosterone, HSD3B
INTRODUCTION
The postnatal pattern of testicular testosterone secretion in highly evolved primates is comprised of a phase of elevated secretion during the first few months of infancy that is followed by a hypoandrogenic state, which in turn persists for the remainder of pre-pubertal development and is terminated by reinitiation of testicular testosterone release at the onset of puberty (Plant et al, 2005; Witchel and Plant, 2014). The childhood/juvenile hiatus in testicular testosterone secretion lasts for approximately 2 years in the monkey and 10 years in man (Plant et al, 2005; Witchel and Plant, 2014). This characteristic postnatal time course of testicular testosterone secretion in higher primates is driven by a corresponding developmental pattern of pituitary LH secretion (Plant et al, 2005; Witchel and Plant, 2014), and correlates with morphological and immunohistochemical characteristics of Leydig cells examined at the light and ultrastructural level (Mancini et al, 1963; Fouquet et al., 1984; Prince, 1984; Fouquet and Raynaud, 1985; Nistal et al, 1986; Prince, 1990; Pelletier et al, 1992).
The origins of the Leydig cell population responsible for testosterone secretion during infancy and puberty in primates are incompletely understood. Following birth, a neonatal population of Leydig cells appears, and in macaques, maximal numbers of neonatal Leydig cells are attained at around 2–3 months of age (Fouquet et al, 1984; Prince, 1990). This cohort is posited to arise by one of two mechanisms. First, some of the regressed fetal Leydig cells that were earlier responsible for synthesis of the testosterone that underlies prenatal masculinization of the male may be reactivated following birth (Mancini et al, 1963; Codesal et al, 1990; Prince 2001; Svechnikov et al, 2010). Second, an additional wave of precursor cells may differentiate into Leydig cells following birth (Prince 2001; Svechnikov et al, 2010). Both mechanisms for generating the neonatal Leydig cell population are considered to be the result of elevated LH secretion at this early stage of postnatal development. Neonatal Leydig cells, like those of the fetus, express both LH receptor (LHCGR) and steroidogenic enzymes such as 3β-hydroxy steroid dehydrogenase, HSD3B (Saez, 1994, Benton et al, 1995; Chen et al, 2009). With the onset of the hypogonadotropic state of the juvenile stage of development, the neonatal Leydig cells lose their steroidogenic characteristics, including the expression of HSD3B, and become quiescent (Saez, 1994; Benton et al, 1995; Chen et al, 2009). These regressed cells are considered to form the population of “immature” Leydig cells of the juvenile (Saez, 1994; Benton et al, 1995; Chen et al, 2009). At the onset of puberty, mitotic activity in the interstitium of the primate testis is low and therefore the appearance of mature Leydig cells at this stage of development presumably results primarily from differentiation of the immature Leydig cells of the juvenile or recruitment of precursor cells (Fouquet and Raynaud, 1985; Amat et al, 1986; Fouquet and Kann, 1987; Berensztein et al, 2002; Haider, 2004). Regardless of their origin these Leydig cells are responsible for the pubertal increase in testicular testosterone secretion that results in elevated concentrations of the circulating steroid and in initiation of spermatogenesis (Saez, 1994; Benton et al, 1995; Chen et al, 2009; Svechnikov et al., 2010).
The purpose of the present study was twofold. First, to systematically examine the time course of the emergence of Leydig cells in the interstitium during spontaneous puberty in the rhesus monkey, a representative highly evolved primate. Second, to examine whether the rise in FSH secretion at the time of puberty contributes to the generation of the adult population of Leydig cells in this Old world monkey. Two studies were performed. In the first, monkeys at defined stages of pubertal development were given a bolus iv injection of BrdU and then castrated 2 – 3 hours later. Testicular tissue was fixed and prepared for immunohistochemical analysis of HSD3B staining and for assessment of S-phase labeling in the interstitium. In the second, juvenile monkeys were stimulated for 11 days with LH and FSH, either alone or in combination, to examine the relative role of the two gonadotropins in the development of the adult population of Leydig cells.
MATERIALS AND METHODS
Tissue
Five μm sections (mounted on Superfrost Plus glass slides, Fisher Scientific, Pittsburgh, PA, USA) of Bouins fixed, paraffin embedded testes that had been generated in three previously published studies (Ramaswamy et al 2000; Simorangkir et al 2003 and 2012) were used.
In the first experiment, which examined Leydig cell development during spontaneous puberty, testis sections from mid-juvenile (15.2±1.08 months of age; 2.9±0.25 kg body wt., mean±SD), late-juvenile (34.2±0.97 months; 4.2±0.68 kg), early-pubertal (42.1±2.73 months; 5.5±0.40 kg) and mid-pubertal (49.0±2.00 months; 7.4±1.02 kg) monkeys (N=3/per group) were used. As reported previously (Simorangkir et al, 2012), late-juveniles had a combined testis volume of <5 ml with no evidence of increased nighttime circulating testosterone levels. Early-pubertal animals had a combined testis volume of 5 ml and nighttime testosterone concentration in excess of 1 ng/ml for several weeks. Mid-pubertal animals had a combined testis volume of at least 15 ml and nighttime testosterone concentration in excess of 1 ng/ml for >10 weeks. For the mid-juvenile group, testicular volume and circulating testosterone concentrations were not determined (Simorangkir et al, 2003). Five μm sections of Bouins fixed paraffin embedded testes from adults (N=3; 8.1±1.95 years of age; 10.9±2.22 kg) that had been previously deposited in our tissue bank were also used. All animals in this experiment had been injected with an iv bolus of BrdU (33 mg/kg BW) 2 to 3 h before castration (Simorangkir et al 2003 and 2012).
In the second experiment, which examined the role of LH and FSH in the generation of the pubertal population of Leydig cells in the monkey, testis sections from the following 4 groups of juvenile males (n=4/group) were used. Animals in Group 1 had received recombinant human FSH (2 IU/kg every 3h for 11 days); Group 2 recombinant single chain human LH (3 IU/kg every 3h for 11 days); Group 3 combined gonadotropin treatment (doses and duration same as used for individual treatments) and Group 4 vehicle (see Ramaswamy et al 2000 for details). The monkeys were 16–19 months of age, and weighed between 2.2–3.7 kg (Ramaswamy et al 2000). All treatments were administered by intermittent iv infusion via an indwelling catheter while animals were maintained in specialized cages that permitted continuous access to the venous circulation as previously described (Ramaswamy et al 2000). At the termination of the 11 days of gonadotropin stimulation or vehicle administration, the monkeys were bilaterally castrated. The bioactivity of both the LH and FSH preparation was confirmed by the finding that treatment with each gonadotropin, alone, resulted in a significant increase in seminiferous cord diameter and in the number of Sertoli cells per testis (Ramaswamy et al 2000). In addition, treatment with LH, either alone or in combination resulted in a dramatic increase in testosterone secretion. Mean circulating concentrations of testosterone in the vehicle, FSH, LH and FSH+LH groups were 0.21±0.06, 0.24±0.12, 4.64±1.9 and 7.18±1.6 ng/ml (mean±SD), respectively, at 60 min following a gonadotropin or vehicle pulse on the last day (day 11) of each of the four treatments (Ramaswamy et al 2003).
For both experiments, the animals were maintained under a controlled photoperiod (lights on, 07.00–19.00 hours), and the experimental protocols were conducted in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals and with approval from the Institutional Animal Care and Use Committee (Ramaswamy, 2000; Simorangkir et al 2003 and 2012).
Immunohistochemistry
Testis sections were first deparaffinized and rehydrated in xylene (2 × 5 min) and ethanol (100%; 2 × 5 min, 95%; 1 × 3 min, and 70%; 1 × 3 min), respectively. After rinsing with 50mM PBS (1 × 2 min) the sections were transferred to 3% hydrogen peroxide in distilled water for 15 min. After repeated rinsing with 50mM PBS (3 × 10 min) non-specific binding was blocked with 5% normal goat serum blocking buffer (NGS, in 50mM PBS with 0.5% Triton X-100) for 60 min at room temperature. The sections were than incubated overnight at 4°C with a rabbit polyclonal primary antibody against synthetic human HSD3B (N terminal amino acids 1–50 of human HSD3B2) (Abcam, ab80500, Cambridge, MA, USA) diluted 1:500 in 5% NGS blocking serum buffer. After rinsing with 50 mM PBS (3 × 10 min) the sections were incubated with a goat anti-rabbit biotinylated secondary antibody (BA-1000, Vector Laboratories Inc, Burlingame, CA, USA) diluted 1:200 in 5% NGS blocking serum buffer for 1 hour at room temperature. The sections were rinsed with 50 mM PBS (3 × 10 min) followed by incubation with AB-complex diluted 1:1000 in PBS (PK-6100, Vectastain ABC kit elite, Vector Laboratories) for 1 hour at room temperature. Thereafter the sections were again rinsed in 50mM PBS followed by chromogen development with 3, 3-diaminobenzidine (DAB, diluted 30:1000; ImmPACT DAB peroxidase substrate kit, SK-4105, Vector Laboratories). The development of chromogen was observed under the microscope to determine the specificity of staining and the reaction was terminated by washing the sections in Milli-Q water. Dark brown coloring indicated the presence of HSD3B in the cytoplasm. Sections, after rinsing with Milli-Q water, were then counterstained with hematoxylin or periodic acid-Schiff’s (PAS)- hematoxylin (Sigma-Aldrich, Inc, St. Louis, MO, USA) and washed with water for 10 min and Milli-Q water for 5 min. After dehydration through graded series of alcohol and clearing in xylene, the sections were coverslipped with Permount mounting medium (Fisher Scientific, Fair Lawn, NJ, USA). For controls the primary antibody was omitted or replaced with normal rabbit serum (Figure 1).
Figure 1.
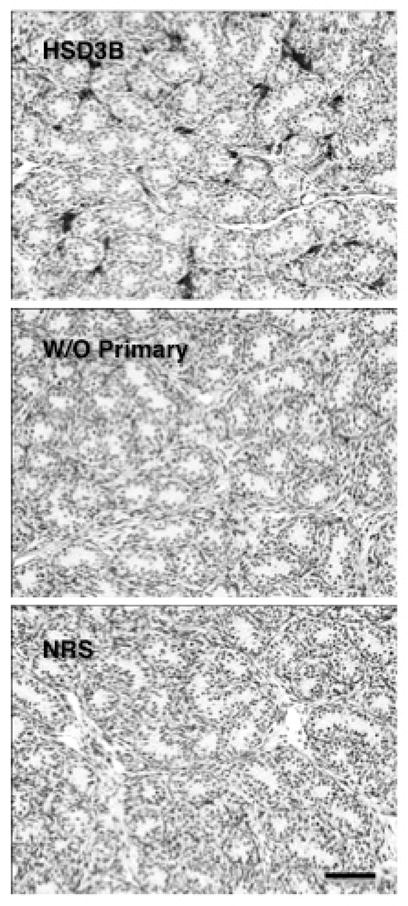
Control profiles of HSD3B immunoexpression in sections of testis from a juvenile rhesus monkey stimulated with LH and FSH in the presence (dark interstitial staining, top panel) or absence of the primary antibody (middle panel) or when primary antibody was substituted with normal rabbit serum (bottom panel). Scale bar, 50 μm.
For the analysis of S phase (BrdU) labeling of interstitial cells, sections from the spontaneous puberty study (Simorangkir et al, 2012) that were stained for BrdU were examined. Briefly, the primary antibody (mouse monoclonal anti-BrdU antibody, diluted 1:33; Roche Diagnostic Corp, Indianapolis, IN, USA) and the secondary antibody (biotinylated horse anti-mouse antibody, diluted 1:200; Vector Laboratories Inc, Burlingame, CA, USA) were used followed by treatment with Vector ABC kit and DAB kit to detect BrdU labeled cells in testis sections. The sections were counterstained with PAS-hematoxylin as described previously (Simorangkir et al, 2012).
Morphometric Analysis
For the enumeration of Leydig cells per testis, the volume fraction of HSD3B positive cells was determined by the point-counting method described previously (Marshall and Plant, 1996, Ramaswamy, 2005). Briefly, a grid of intersecting lines, using a 20×20 eyepiece graticule, was superimposed over sections to be analyzed. Twenty five randomly chosen fields were examined at 40X magnification and the number of test points falling over the nuclei of HSD3B positive cells were counted and converted to a percentage of the total points per animal. Nuclear diameter of HSD3B positive cells was calculated from the average of 2 perpendicular measurements of each nucleus, and mean diameter of 25 nuclei from each animal was determined. Leydig cell number per testis was then calculated using the values for Leydig cell nuclear volume and testis volume, the latter obtained by dividing the testis weight by 1.04: the specific gravity previously established for testis of the adult monkey (Marshall and Plant, 1996).
Proliferation of interstitial cells throughout peripubertal development (Experiment 1) was determined by examining S-phase cells in the testis that had incorporated BrdU in the DNA following an iv bolus of the nucleotide 2–3 h prior to castration.
Statistical Analysis
Data are expressed as the mean ± SD. Significant differences (P < 0.05) were determined using one-way ANOVA followed by Neuman-Keuls post hoc test for multiple comparisons.
RESULTS
Experiment 1
With the exception of the mid juveniles, robust HSD3B immunostained cells were observed in the interstitium of all testes collected at all developmental stages (Figure 2). The mean number of Leydig cells per testis in the adult group was approximately 163 × 106, which was at least fourfold greater than that for the other groups (Figure 3). The mean number of HSD3B positive cells/gram of testis was 0.23 × 106 in late juveniles and this declined to approximately 0.05 × 106 at the later developmental stages. Nuclear diameter of HSD3B immunostained cells in testes from pubertal animals was significantly greater than that for late juveniles (Figure 3). The transition into adulthood, however, was not associated with a further increase in this parameter.
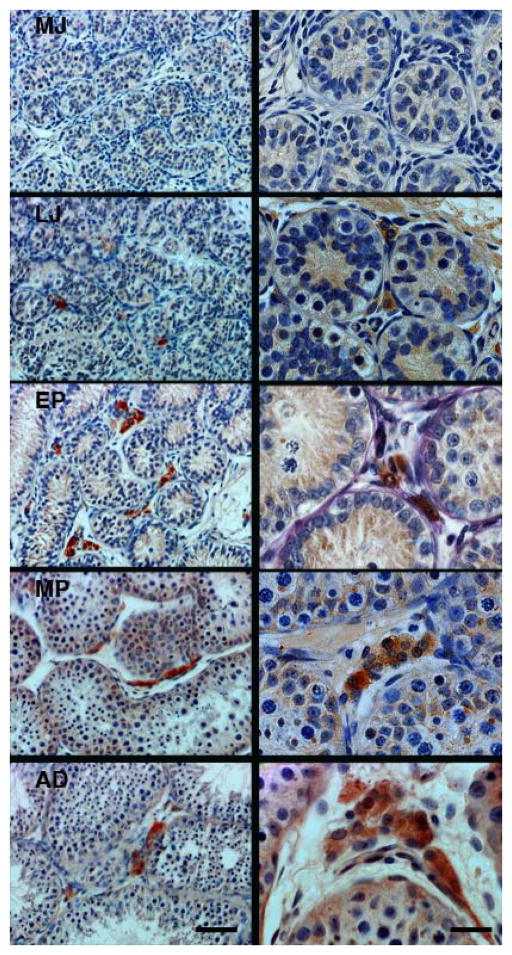
Figure 2.
Representative profiles of HSD3B immunoexpression, used as a marker of Leydig cells, in the interstitium of the testis during spontaneous puberty in the rhesus monkey. Note HSD3B immunoactivity was absent in the testis of mid-juvenile (MJ) animals, while the first appearance of the enzyme was evident in late-juvenile (LJ) animals followed by robust expression in the early-pubertal (EP), mid-pubertal (MP) and adult (AD) stages of development. Scale bars, 50 μm (left-hand panels) and 20 μm (right-hand panels).
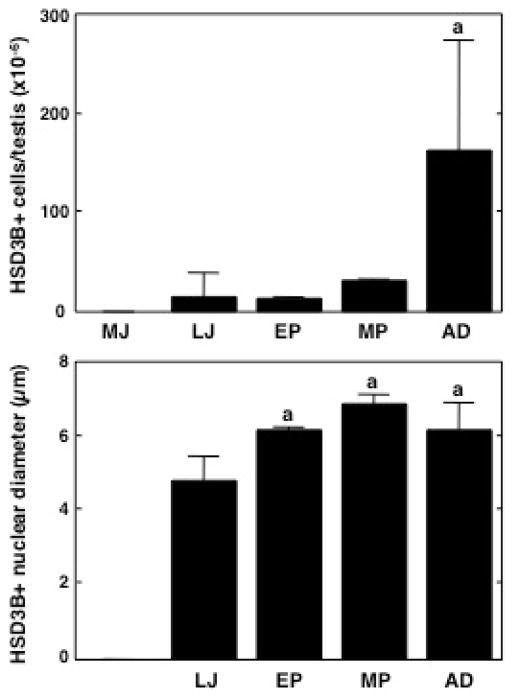
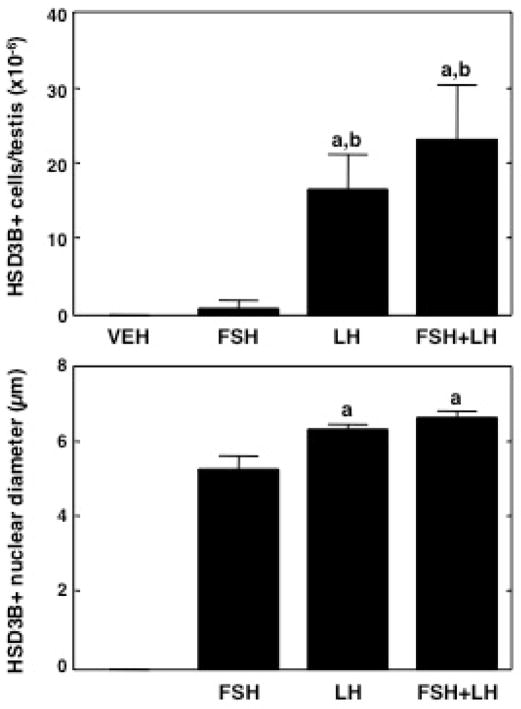
Figure 3.
Number of Leydig cells (HSD3B immunopositive)/testis (top panel), and corresponding changes in their nuclear diameter (bottom panel) during peri-pubertal development in the rhesus monkey. While HSD3B positive cells were absent in the mid-juvenile (MJ) group, their number gradually increased during subsequent pubertal development resulting in a marked elevation in adulthood (AD) (top panel). A significant increase in nuclear diameter occurred during the late-juvenile (LJ) - early pubertal (EP) transition but stabilized thereafter (bottom panel). Note, the nuclear diameter was not determined in the MJ group (bottom panel) since HSD3B immunopositive cells were absent. MP, mid-pubertal. a, P≤ 0.05 from the remaining groups.
In contrast to cells within seminiferous tubules, BrdU labeled interstitial cells were very rarely observed in testes from any stage of development (Figure 4). A systematic screening of a least 2 slides from a testis of each monkey at each stage of development revealed at most no more than 4 labeled interstitial cells in the sections examined from any one animal. This parameter therefore was not formally quantified.
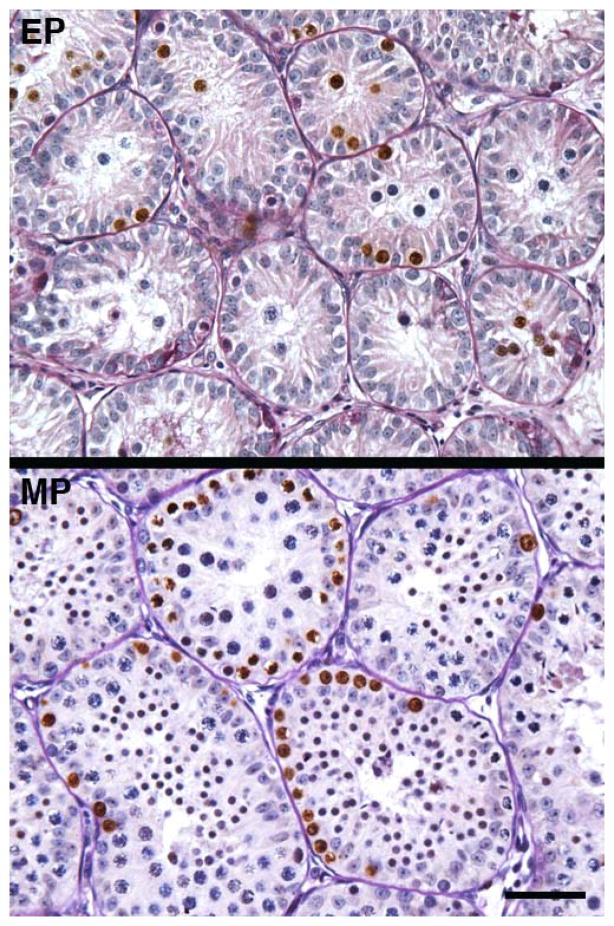
Figure 4.
Profiles of BrdU labelled nuclei (brown) in the testis of an early pubertal (EP) and mid pubertal (MP) rhesus monkey. Note the absence of BrdU label in the interstitium, which is in striking contrast to robust S-phase labelling of cells within the seminiferous tubules. The absence of BrdU labeling in the interstitium from testes of EP and MP monkeys may be compared to the robust HSD3B signal observed at these stages of development and shown in Figure 2. Scale bar, 50 μm. For photomicrographs illustrating the typical architecture of the testis under control conditions and the stimulation paradigm the reader is referred to Ramaswamy et al 2000.
Experiment 2
Consistent with the findings in Experiment 1, HSD3B immunostained cells were not observed in the interstitium of testes collected from vehicle treated juvenile monkeys (Figure 5). Immunopositive interstitial cells were unequivocally identified in the three hormone treated groups of juvenile animals, and this staining was robust following 11 days of stimulation with LH either alone or in combination with FSH (Figure 5). The mean number of interstitial cells per testis in the two LH treated groups were not different between each other but both were greater by at least an order of magnitude than that observed following stimulation with FSH, alone (Figure 6). Interestingly, FSH alone also resulted in the definitive appearance of HSD3B immunostained cells (Figure 5) but this effect was not significant (Figure 6). Nuclear diameter of HSD3B immunostained cells in testes from LH stimulated juveniles was greater than that in FSH stimulated animals (Figure 6).
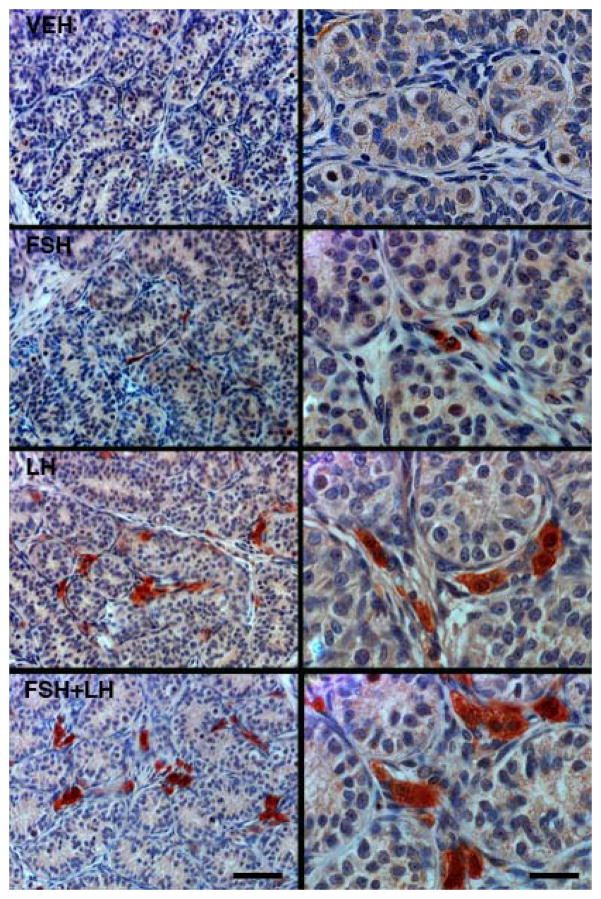
Figure 5.
Representative profiles of interstitial HSD3B immunoexpression (a Leydig cell marker) in testes of juvenile monkeys treated for 11 days with FSH, LH or FSH+LH. Note the absence of HSD3B immunostaining in a control vehicle (VEH) treated animal (top panels). As expected, stimulation with LH, either alone or in combination with FSH, resulted in robust and dramatic increase in the expression of this marker. Interestingly, FSH alone also upregulated HSD3B, although to a considerably lesser extent than that of LH. Scale bars, 50 μm (left-hand panels) and 20 μm (right-hand panels).
Figure 6.
Number of Leydig cells (HSD3B immunopositive)/testis (top panel), and corresponding changes in their nuclear diameter (bottom panel) in juvenile monkeys treated for 11 days with FSH, LH or FSH+LH. HSD3B positive cells were absent in the vehicle (VEH) treated group. Treatment with LH, either alone or in combination with FSH, resulted in a significant increase in the number of Leydig cells (top panel). Interestingly, FSH was able to also stimulate the expression of this enzyme although the effect was not statistically significant by ANOVA (top panel). A significant increase in nuclear diameter of Leydig cells was noted with LH stimulation, either alone or in combination with FSH (bottom panel). Note the nuclear diameter was not determined in the VEH group (bottom panel) since HSD3B immunoactivity was absent. a, P ≤0.05 from the remaining groups and b, from FSH group.
Discussion
The present study provides a systematic examination of the emergence of adult Leydig cells during spontaneous puberty in a representative highly evolved primate, the rhesus monkey, and of the relative role of LH and FSH in triggering this important developmental event in the male gonad. The absence of HSD3B positive cells in the testis of the mid juvenile group in the present study confirms the view that the prepubertal primate testis contains few, if any, recognizable Leydig cells (Fouquet et al., 1984 Liang et al., 1999) and is consistent with the finding that testicular testosterone secretion during this phase of primate development is minimal (Steiner and Bremner, 1981; Fouquet et al., 1984, Plant 1985; Liang et al., 1999; Witchel and Plant, 2014). The expansion of the pubertal population of Leydig cells was associated with a concomitant increase in nuclear diameter that became first apparent in the mid-pubertal group. In the rat, the nuclear diameter of mature adult-type Leydig cells is also greater when compared to immature adult-type cells, a developmental change that is probable indicative of Leydig cell maturation (Chen et al., 2009; Zirkin, 2010).
In the rhesus monkey, the maximal increase in the number of Leydig cells occurred between mid puberty and adulthood and was directly related to the overall growth of the testis. As the pubertal testis grows, the volume fraction of the seminiferous tubules increases and the number Leydig cells per unit mass decreased. Similar observations have been reported for the closely related cynomolgus macaque, Macaca fasiculata, and in both species the number of Leydig cells in the adult testis appear essentially identical (Fouquet et al., 1984). The protracted time course in the pubertal expansion of Leydig cells in the rhesus macaque is in striking contrast to that previously reported for the corresponding developmental proliferation of Sertoli cells, which is an early and relatively rapidly completed event in this species of macaque (Simorangkir et al 2012).
The finding of HSD3B positive cells in the late juvenile monkeys is surprising because circulating testosterone, as assessed in weekly nocturnal blood samples, had not yet begun to rise in this group (Simorangkir et al 2012). This contradiction most likely reflects an inadequacy of the weekly sampling frequency employed in the present study to detect the initial activation of a pulsatile mode of testicular testosterone secretion at the onset of puberty. If this is the case, the late juvenile animals should be considered as a “very early” pubertal group. This view is consistent with our earlier finding that an occasional differentiating spermatogonia was observed in two of the animals in this group (Simorangkir et al 2012),
The finding that S-phase interstitial cells, as identified by BrdU labeling, were observed only very rarely supports the view that the appearance of Leydig cells at the onset of puberty in primates results from differentiation of the immature Leydig cells or recruitment of precursor cells (Fouquet and Raynaud, 1985; Amat et al, 1986; Fouquet and Kann, 1987; Berensztein et al, 2002). This being the case, it might be predicted that the total number of interstitial cells in the juvenile and adult monkey would be similar.
That the initial phase of the pubertal expansion of the Leydig cell population is driven primarily by the increase in LH secretion at this stage of development was indicated by the finding that in mid juvenile monkeys, precocious stimulation with LH, either alone or in combination with that of FSH, resulted in a 20 to 30 fold increase in the mean number of HSD3B positive cells and a significant increase in their nuclear diameter.
Interestingly, precocious FSH stimulation, alone, also resulted in the appearance of HSD3B positive cells. The mean number of these cells per testis, however, was not significantly increased by 11 days of FSH stimulation, and as previously reported by this laboratory there was no effect on plasma testosterone when assessed on day 8 of FSH stimulation (Ramaswamy et al. 2000). An action of a monotropic FSH stimulation of immature Leydig cells has been previously reported for non-primate species. In immature, hypophysectomized rats treatment with partially or highly purified rat FSH was found to induce Leydig cell hypertrophy, hyperplasia, expression of LHCGR and steroidogenic activity (Kerr and Sharpe, 1985, Teerds et al., 1989a). A more recent study of the gonadotropin-deficient hypogonadal (hpg) mouse also shows an increase in Leydig cell number after treatment with recombinant FSH. (O’Shaughnessy et al., 2010).
In addition, a synergistic action of FSH and LH on testicular steroidogenesis, first demonstrated with the perfused rabbit testes (Johnson and Ewing, 1971), has been reported for the adult rhesus monkey (Simorangkir et al, 2009) when recombinant human gonadotropins were administered in a pulsatile mode of for 4 weeks to animals in which endogenous gonadotropin secretion had been blocked by treatment with a GnRH receptor antagonist. In contrast, twice daily injections of human FSH to adult cynomolgus monkey (Macaca fascicularis) did not affect either Leydig cell number or testosterone production (Teerds et al 1989b).
Since it is generally accepted that testicular expression of the FSH receptor is restricted to the Sertoli cell (Simoni et al, 1997) any action of FSH on the Leydig cell is likely to be mediated by paracrine signals from the Sertoli cell. In this regard, FSH stimulated testosterone production in co-cultures of porcine Sertoli cells and Leydig cells, but not when Leydig cells were cultured alone (Benahmed et al., 1985).
In summary the present study of the rhesus monkey, provides a systematic description of the expansion in the Leydig cell population at the time of puberty in this macaque, and provides evidence that LH signaling is the major gonadotropin drive to this developmental event.
Acknowledgments
This work was supported by National Institutes of Health grants U54HD08610, R01HD013254 and R01HD072189 (TMP). We thank Ms. Rachel Roslund for assisting with the immunohistochemistry. IV was supported by Fundatie van de Vrijvrouwe van Renswoude, The Netherlands, for work performed at TMP’s laboratory in Pittsburgh, USA.
Footnotes
Authors’ roles
IV performed immunohistochemistry, collated data, analyzed results and contributed to writing the manuscript.
SR contributed to the design of the study and writing the manuscript, supervised IV, performed immunohistochemistry, analyzed the results, collated and prepared the figures.
KJT and JK contributed to study design, and served as mentor to IV.
TMP led the design of the study and writing the manuscript, directed analyses of data and preparation of the figures, and served a co-mentor to IV.
References
- Amat P, Paniagua M, Nistal M, Martin A. Mitosis in adult human Leydig cells. Cell Tissue Res. 1986;243:219–221. doi: 10.1007/BF00221871. [DOI] [PubMed] [Google Scholar]
- Benahmed M, Reventos J, Tabone E, Saez JM. Cultured Sertoli cell-mediated FSH stimulatory effect on Leydig cell steroidogensis. Am J Physiol. 1985;248:176–181. doi: 10.1152/ajpendo.1985.248.2.E176. [DOI] [PubMed] [Google Scholar]
- Benton L, Shan L-X, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Molec Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Berensztein EB, Sciara MI, Rivarola MA, Belgorosky A. Apoptosis and proliferation of human testicular somatic and germ cells during prepuberty: High rate of testicular growth in newborns mediated by decreased apoptosis. JCEM. 2002;87:5113–5118. doi: 10.1210/jc.2002-020032. [DOI] [PubMed] [Google Scholar]
- Chen H, Ge R-S, Zirkin BR. Leydig cells: From stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codesal J, Regadera J, Nistal M, Regadera-Sejas J, Paniagua R. Involution of human fetal Leydig cells. An immunohistochemical, ultrastructural and quantitative study. J Anat. 1990;172:103–114. [PMC free article] [PubMed] [Google Scholar]
- Fouquet J-P, Kann M-L. Mitotic activity in monkey and rat Leydig cells. Reprod Nutr Develop. 1987;27:1053–1062. doi: 10.1051/rnd:19870808. [DOI] [PubMed] [Google Scholar]
- Fouquet J-P, Raynaud F. Renewal of Leydig cells in the neonatal and adult monkey: a radioautographic study. Biol Cell. 1985;54:187–190. doi: 10.1111/j.1768-322x.1985.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Fouquet J-P, Meusy-Dessolle N, Dang DC. Relationship between Leydig cell morphometry and plasma testosterone during postnatal development of the monkey, Macaca fascicularis. Reprod Nutr Develop. 1984;24:281–296. doi: 10.1051/rnd:19840307. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell Biology of Leydig cells in the Testis. Int Rev Cytol. 2204;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Johnson BH, Ewing LL. Follicle-stimulating hormone and the regulation of testosterone secretion in rabbit testes. Science. 1971;173:635–637. doi: 10.1126/science.173.3997.635. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Sharpe RM. Follicle-stimulating hormone induction of Leydig cell maturation. Endocrinology. 1985;116:2592–2604. doi: 10.1210/endo-116-6-2592. [DOI] [PubMed] [Google Scholar]
- Liang J-H, Sankai T, Yoshida T, Cho F, Yoshikawa Y. Localization of immunoreactive testosterone and 3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase in cynomolgus monkey (macaca fascicularis) testes during postnatal development. J Med Primatol. 1999;28:62–66. doi: 10.1111/j.1600-0684.1999.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Mancini RE, Vilar O, Lavieri JC, Andrada JA, Heinrich JJ. Development of Leydig cells in the normal human testis: A cytological, cytochemical and quantitative study. Am J Anat. 1963;112:203–214. [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- Nistal M, Paniagua R, Regadera J, Santamaria L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res. 1986;246:229–236. doi: 10.1007/BF00215884. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotropin-deficient hypogonadal mice lacking androgen receptor. Reproduction. 2010;139:177–184. doi: 10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Simard DJ, Luu-The V, Belanger A, Labrie F. Ontogeny and subcellular localization of 3β-hydroxysteroid dehydrogenase (3β-HSD) in the human and rat adrenal, ovary and testis. J Steroid Biochem Molec Biol. 1992;43:451–467. doi: 10.1016/0960-0760(92)90084-v. [DOI] [PubMed] [Google Scholar]
- Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Simorangkir DR, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann NY Acad Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016. [DOI] [PubMed] [Google Scholar]
- Prince FP. Ultrastructure of immature Leydig cells in the human prepubertal testis. Anat Rec. 1984;209:165–176. doi: 10.1002/ar.1092090204. [DOI] [PubMed] [Google Scholar]
- Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat Rec. 1990;228:405–417. doi: 10.1002/ar.1092280406. [DOI] [PubMed] [Google Scholar]
- Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol. 2001;168:213–216. doi: 10.1677/joe.0.1680213. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S. Pubertal augmentation in juvenile rhesus monkey testosterone production induced by invariant gonadotropin stimulation is inhibited by estrogen. JCEM. 2005;90:5866–5875. doi: 10.1210/jc.2005-0092. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious Sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta) Biol Reprod. 2000;63:82–88. doi: 10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle –stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta) Endocrinology. 2003;144:1175–1185. doi: 10.1210/en.2002-221078. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: Endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Plant TM. Sertoli cell proliferation during prepubertal development in the rhesus monkey (Macaca mulatta) is maximal during infancy when gonadotropin secretion is robust. JCEM. 2003;88:4984–4989. doi: 10.1210/jc.2002-021858. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Ramaswamy S, Marshall GR, Pohl CR, Plant TM. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta) Human Reprod. 2009;24:1584–1595. doi: 10.1093/humrep/dep052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simorangkir DR, Ramaswamy S, Marshall GR, Roslund R, Plant TM. Sertoli cell differentiation in rhesus monkey (Macaca mulatta) is an early event in puberty and precedes attainment of the adult complement of undifferentiated spermatogonia. Reproduction. 2012;143:513–522. doi: 10.1530/REP-11-0411. [DOI] [PubMed] [Google Scholar]
- Steiner RA, Bremner WJ. Endocrine correlates of sexual development in the male monkey, Macaca fascicularis. Endocrinology. 1981;109:914–919. doi: 10.1210/endo-109-3-914. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Landreh L, Weisser J, Izzo G, Colon E, Svechnikova I, Soder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Closset J, Rommerts FFG, de Rooij DG, Stocco DM, Colenbrander B, Wensing CJG, Hennen G. Effects of pure FSH and LH preparations on the number and function of Leydig cells in immauture hypophysectomized rats. J Endocrinol. 1989a;120:97–106. doi: 10.1677/joe.0.1200097. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Rommerts FFG, Van de Kant HJG, de Rooij DG. Leydig cell number and function in the adult cynomolgus monkey (Macaca fascicularis) is increased by hCG treatment but not by daily FSH treatment. J Reprod Fert. 1989b;87:141–146. doi: 10.1530/jrf.0.0870141. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Plant TM. Puberty: Gonadarche and Adrenarche. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology. Saunders Elsevier; Philadelphia: 2014. pp. 377–421. [Google Scholar]
- Zirkin BR. Where do adult Leydig cells come from? Biol Reprod. 2010;82:1019–1020. doi: 10.1095/biolreprod.110.084111. [DOI] [PMC free article] [PubMed] [Google Scholar]