Abstract
Background
Administration of topical rapamycin (RPM) suppresses the regeneration and revascularization of photocoagulated blood vessels induced by pulsed dye laser (PDL).
Objective
To systematically elucidate the molecular pathophysiology of the inhibition of PDL-induced angiogenesis by topical RPM in a rodent model.
Methods
The mRNA expression profiles of 86 angiogenic genes and phosphorylation levels of ribosomal protein S6 kinase (P70S6K) in rodent skin were examined with or without topical RPM administration post-PDL exposure.
Results
The PDL-induced systematic increases in transcriptional levels of angiogenic genes showed a peak expression at days 3 to 7 post-PDL in rodent skin. Topical application of 1% RPM significantly and systematically suppressed the PDL-induced increase in mRNA levels of the examined angiogenic genes during the first five days post-PDL. The phosphorylation levels of P70S6K increased after PDL exposure but those increases were suppressed by the topical application of RPM. After topical application, RPM penetrated to an approximate depth of 768.4 µm into rodent skin.
Conclusion
Topical application of 1% RPM can significantly and systematically suppress the PDL-induced early stage of angiogenesis via inhibition of the AKT/mTOR/P70S6K pathway in a rodent model.
Keywords: Pulsed dye laser, port wine stain, rapamycin, angiogenesis, P70S6K
INTRODUCTION
Port wine stain (PWS) is a congenital, progressive vascular malformation of human skin involving the superficial vascular plexus. PWS occurs in an estimated 3–5 children per 1,000 live births [1–3]. Approximately 1,500,000 individuals in the United States and thirty-two million people worldwide have PWS birthmarks [4–6]. Since most malformations occur on the face, PWS is a clinically significant problem in the majority of patients. Personality development is adversely influenced in virtually all patients by the negative reaction of others to a "marked" person. Detailed studies have documented lower self-esteem in such patients and problems with interpersonal relationships [7–9]. Studies have indicated a high level of psychological morbidity in PWS patients resulting from feelings of stigmatization that are frequently concealed in casual social interactions.
In childhood, PWS are flat red macules, but lesions tend to darken progressively to purple and, by middle age, often become raised as a result of the development of vascular nodules [10,11]. Recently, a low frequency allelic mutation (c.548G→A, p.R183Q) in the Guanine nucleotide-binding protein, G alpha subunit q (GNAQ), has been identified in PWS skin specimens [12]. We have also found consecutive activation of the c-Jun N-terminal kinases (JNK) and extracellular signal regulated kinases (ERK) in both infantile and adult PWS [13]. Taken altogether, these studies have begun to elucidate the molecular mechanisms underlying the pathogenesis PWS.
The pulsed dye laser (PDL) is the current treatment of choice for PWS [14,15]. However, the degree of PWS blanching achieved following PDL can be variable and unpredictable with an average treatment success rate below 10%, if the ultimate standard required is complete blanching of the lesion, due to blood vessel recurrence [16–18]. The regeneration and revascularization of blood vessels post-PDL treatment is a critical barrier that must be overcome in order to achieve an adequate PWS therapeutic outcome [19]. In this study, we hypothesize that PDL combined with the administration of topical rapamycin (RPM), an angiogenesis inhibitor, can suppress PDL-induced angiogenesis in rodent skin, thus providing a potential treatment for PWS. RPM can inhibit the mammalian target of rapamycin (mTOR) activity by forming a complex with FK-binding protein 12 (FKBP12) which binds to mTOR directly, thus suppressing the AKT/mTOR/P70S6K angiogenic pathway [20–23]. In previous studies, we have demonstrated that RPM can: (1) reduce regeneration and revascularization of blood vessels [24]; (2) suppress the PDL-induced increase in mRNA and protein levels of hypoxia-inducible factor-1 alpha (HIF-1α), vascular endothelial growth factor (VEGF) and ribosomal protein S6 kinase (P70S6K), as well as the phosphorylation levels of P70S6K and AKT on day 3 post-PDL exposure [19]; (3) abolish the upregulation of certain “stem cell” antigens, such as nestin, thereby interrupting the vascular repair process induced by PDL exposure [25]; and (4) based on a case report, enhance the blanching response in a PWS patient when RPM was given systematically post-PDL [26]. These previous studies have demonstrated the feasibility and potential of a new therapeutic strategy for PWS. Herein, we attempt to further systematically elucidate the molecular mechanism(s) of PDL-induced angiogenesis and evaluate the inhibitory effects induced by topical RPM on the regeneration and revascularization of photocoagulated blood vessels.
MATERIALS AND METHODS
Animals
All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee, University of California, Irvine. Adult male Sprague Dawley rats with an initial bodyweight of 100–150 grams were used. The preparation of topical RPM has been described previously [19].
Laser Irradiation
Laser exposure was performed on the abdominal side of the rodent skin. Sites were irradiated with a 585 nm PDL (Candela, Wayland, MA); pulse duration was 0.45 ms, energy density was 8 J/cm2 delivered on a 2 mm spot diameter. Each animal had three sites in designated areas (1.5 cm × 2 cm) side by side on the skin: control (no treatment), PDL+vehicle and PDL+RPM. The vehicle was composed of the exact same ointment as the topical RPM formulation, but without RPM. Topical RPM was applied daily for 1, 3, 5, 7, 10 or 14 days post-PDL-exposure. Animals were then euthanized and four biopsy samples (4 mm diameter) were taken from each site. Three out of four biopsy samples from the same treatment site were randomly selected and assigned for mRNA extraction, protein extraction and immunohistochemistry experiments. Three to four animals were allocated for each treatment time point. The sample size justification for the number of samples taken from rodent skin was based on our previous study [19].
RNA Extraction and Real Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Array Analysis
Total RNA was extracted from skin biopsy samples using the RNeasy Mini kit (Qiagen, Carlsbad, CA) according to the manufacturer’s manual. To generate cDNA, 1.0 µg of total RNA was reverse-transcribed in a 20-µl reaction containing 1× RT buffer (Clontech, Mountain View, CA), 0.5 mm dNTPs, 0.5 µg of oligo (dT) 15-mer primer, 20 units of RNasin, and 5 units of SMART Moloney murine leukemia virus reverse transcriptase (Clontech, Mountain View, CA). The RT reaction was carried out at 42°C for 2 hrs. Thereafter, 0.5 µl of each sample (25 ng) was used directly for real time PCR analysis which was performed using the LightCycler System (Roche, Pleasanton, CA). Five house-keeping genes were used as controls to normalize the amplification data: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Rplp1, Hprt1, Rpl13a, and actin-β. The reaction for the multiplex real time PCRs contained 1× SYBR Green qPCR Master Mix (Clontech, Mountain View, CA), 2.6 ng of each template, and 50 nM of each specific primer in a 7-µl total volume in a 384-well format. Each reaction was performed in duplicate under identical conditions. The PCR conditions were one cycle at 95°C for 2 min followed by 45 cycles of 15 s at 95°C and 60 s at 60°C. Relative quantification of the real time PCR was based upon the amplification efficiency of the target and reference genes and the cycle number at which fluorescence crossed a prescribed background level, cycle threshold (Ct).
Immunohistochemistry
Skin biopsies (4 mm) from each site and normal skin were taken from the rodents. Biopsy tissue was fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) and processed for permanent paraffin embedding on an ASP 300 tissue processor (Leica Microsystems, Bannockburn, IL, USA). Approximately 6 µm thick paraffin sections were cut and collected.
The paraffin sections were deparaffinized according to routine procedures. The antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0) at 90°C for 12 hours. The sections were then incubated in a humidified chamber overnight at 4°C with a pP70S6K antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, 1:50 dilution). A biotinylated anti-mouse secondary antibody was incubated with sections for 2 hours at room temperature after the primary antibodies’ reaction. An indirect biotin avidin diaminobenzidine (DAB) system (Dako, Glostrup, Denmark) was used for detection.
Statistical Analysis
The expression level of each gene was normalized to the mRNA levels of the five aforementioned housing keeping genes for all the PCR reactions in each 384-well plate format. At each treatment time point, the fold changes of mRNA levels of each gene in the laser and laser+RPM groups from the same animal were obtained by comparison to the control values which were set as 1. The gene expression data from independent samples which were obtained from different animals (n=3 or 4) at the same treatment time point were then summarized and presented as Mean ± S.D. We treated these fold changes as independent samples drawn from normal (0, sigma^2), i.e., the null hypothesis was that there was no difference among the groups. The group data sets were not normally distributed because these 86 genes were purposely selected for this study. A non-parametric analysis Wilcoxon Signed-Rank test was performed among the groups (control, laser and laser+RPM) at each treatment time point. ANOVA was used to compare expression levels of individual genes among the groups (control, laser and laser+RPM) at each treatment time point. If there was a statistical significance (p<0.05) among the groups, a t-test was further used to compare the expression significance of target genes between the paired groups, e.g., control versus laser and laser versus laser+RPM groups. The bonferroni correction was used to adjust for the inflated type I error in multiple comparisons.
RESULTS
Expression Profiles of Angiogenic Factors Induced by PDL
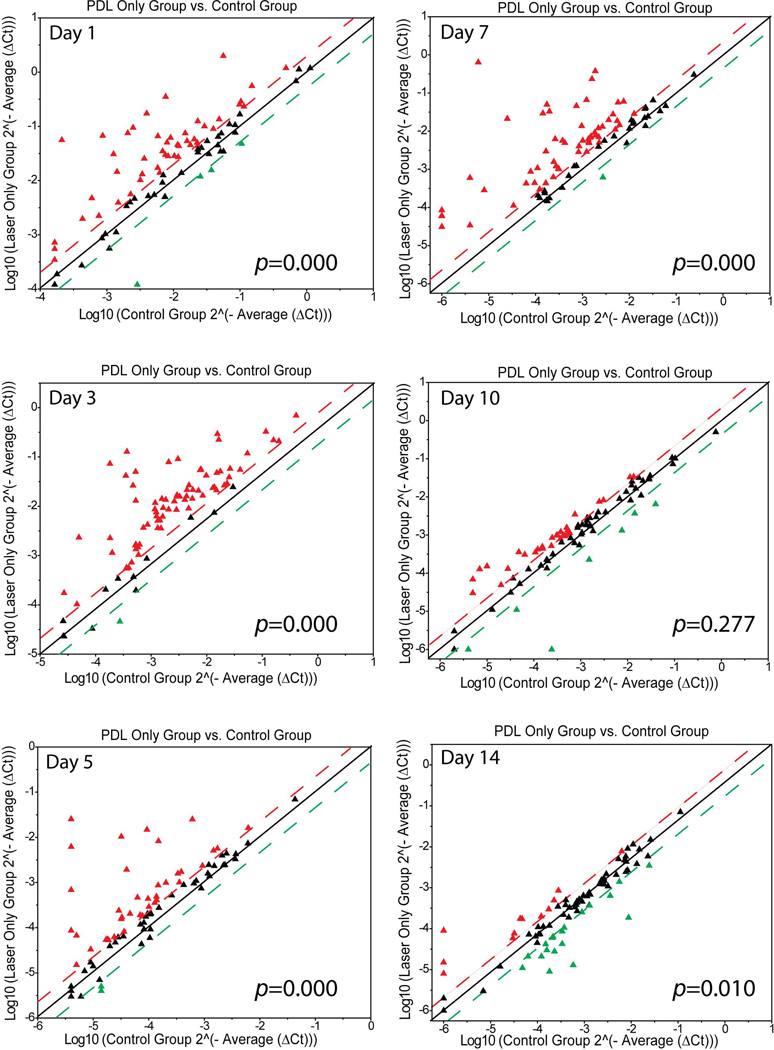
The mRNA levels of 86 angiogenic factors during the post-PDL wound healing process were investigated using the real-time RT-PCR array. At days 1, 3, 5 and 7 post-PDL exposure, the numbers of angiogenic genes with increased mRNA levels by a factor of 2.0 or higher were 46, 57, 48 and 81 out of 86, respectively, as compared to control groups (Figure 1). Most of the angiogenic genes had their peak expressions through days 3 to 7 post-PDL exposure alone. Thereafter, the expression levels started to decline (Figure 1). On days 10 and 14 post-PDL exposure alone, 29 and 12, respectively, angiogenic genes showed their expression levels above the threshold. However the expression levels were much lower as compared to those at days 5 and 7 post-PDL exposure (Figure 1). Statistical analysis showed that there were overall and significant increases in mRNA levels of 86 angiogenic genes in the laser groups on days 1 to 7 post-PDL exposure as compared to controls (table 1). At days 1, 3, 5, 7, 10 and 14 post-PDL exposure, the numbers of angiogenic genes with decreased mRNA levels by a factor of 2 or higher in the PDL only groups were 4, 1, 2, 1, 7 and 10, respectively (Figure 1).
Fig. 1.
Scatter plots showing the expression profiles of 86 angiogenic factors post-PDL exposure. The threshold was set at 2.0 times control. The red triangles above the upper threshold line (red) represent the angiogenic genes in the PDL groups that had increases in their mRNA levels by a factor of 2.0 or more as compared to the normal control group. The green triangles below the lower threshold line (green) represent the angiogenic genes in the PDL groups that had decreases in their mRNA levels by a factor of 2.0 or more as compared to the normal control group. The black triangles between the red and green threshold lines represent the angiogenic genes in the PDL groups that showed either increases or decreases in their mRNA levels 2.0 times as compared to the control group. P values were from the Wilcoxon Signed-Rank test.
Table 1.
Topical RPM Suppressed PDL-induced Expression of Angiogenic Genes in Rodent Skin
| Day / P value |
P1 (Laser vs control) |
P2(laser+RPM vs control) |
P3 (Laser vs laser+RPM) |
|---|---|---|---|
| 1 | 0.000 | 0.000 | 0.000 |
| 3 | 0.000 | 0.569 | 0.000 |
| 5 | 0.000 | 0.000 | 0.000 |
| 7 | 0.000 | 0.000 | 0.000 |
| 10 | 0.277 | 0.738 | 1.000 |
| 14 | 0.010 | 0.000 | 0.000 |
The fold changes of mRNA levels of 86 angiogenic genes were obtained by comparing to the control values which were set as 1. The logarithmic value of the fold change of each gene was used during the statistical analysis. There were n=3 or 4 animals for each treatment time point. P is the value from the Wilcoxon Signed-Rank test among the groups.
Gene family analysis showed that fibroblast growth factors (FGFs), platelet-derived growth factor (PDGF)-b, hepatocyte growth factor (HGF) and VEGF were the most upregulated angiogenic growth factors induced by PDL, indicating activation of the corresponding signaling pathways by PDL. Chemokines and the pro-inflammatory cytokines family, represented by Cxcl2 and interleukin (IL)-6, had increased mRNA levels, suggesting activation of the associated inflammatory pathways. Furthermore, the mRNA levels of the gene family related to degradation of vascular basement membrane and remodeling of the extracellular matrix of the vasculature during angiogenesis, such as matrix metalloproteinase (MMP)-3, 9, 19 and tissue inhibitor of metalloproteinase (TIMP)-1, were dramatically upregulated in PDL-exposed rodent skin.
Inhibitory Effects of Topical RPM on Expression Profiles of Angiogenic Factors Induced by PDL
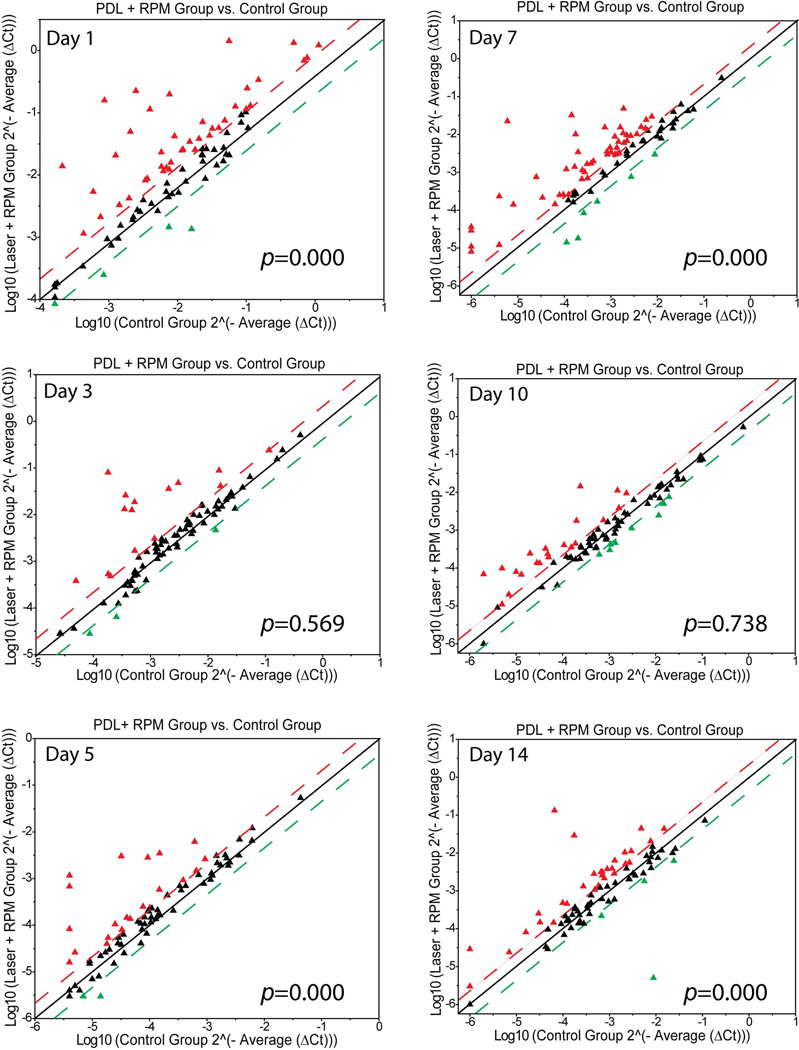
At days 3 and 5 post-PDL exposure, the numbers of angiogenic genes with increased mRNA levels by a factor of 2.0 or higher in PDL+RPM groups declined to 15 and 21, respectively, suggesting that topical RPM rendered a systematic inhibition of the PDL-induced angiogenesis (Figure 2). On day 7 post-PDL exposure, RPM showed certain levels of inhibition on the overall expression levels of angiogenic factors, but this effect was not as profound as that observed on days through 3 to 5 (Figure 2). Statistical analysis showed that topical RPM exhibited an overall and significant suppression of mRNA levels of 86 angiogenic genes in the laser+RPM groups on days 1 to 7 post-PDL exposure as compared to controls (table 1). On day 10 post-PDL exposure, RPM didn’t change the overall expression patterns of these angiogenic genes as compared to the control group (Figure 2, table 1). RPM showed the opposite effect on the overall expression patterns of some angiogenic factors, e.g., expression levels were significantly increased as compared to control, on day 14 post-PDL exposure (Figure 2, table 1). Therefore, RPM exhibited a profound overall inhibition through days 1 to 7 post-PDL exposure, but thereafter the inhibition became less effective.
Fig. 2.
Topical RPM systematically suppressed the PDL-induced early stage of angiogenesis. The threshold was set at 2.0 times control. The red, green and black triangles represent the same fold changes of mRNA levels of angiogenic genes as in Fig.1. P values were from the Wilcoxon Signed-Rank test.
The Expression Profiles of Some Key Angiogenic Factors
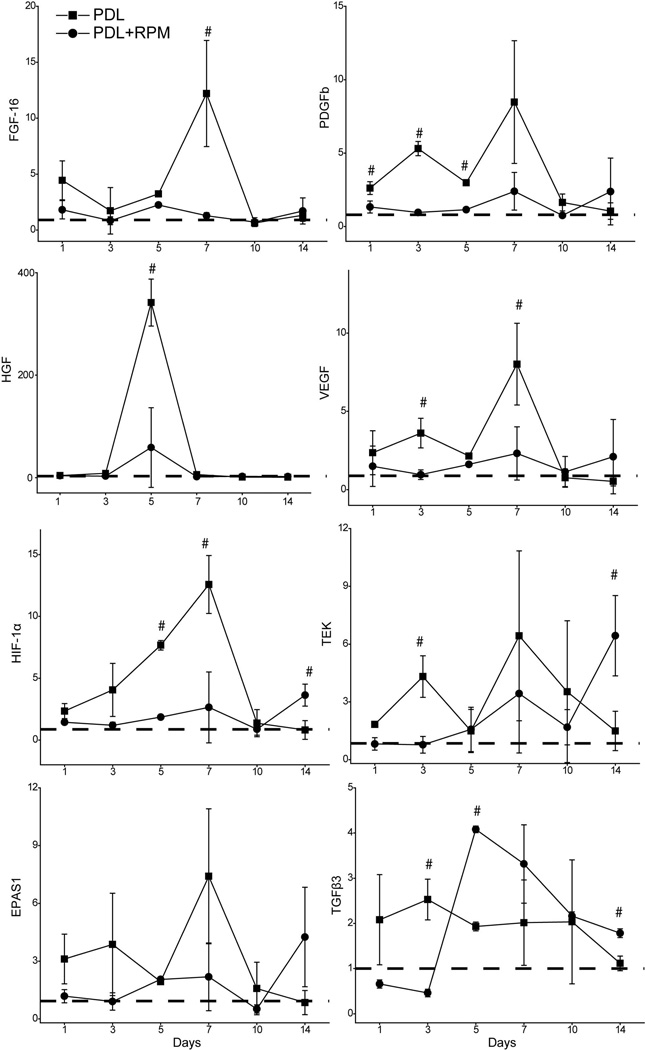
Many growth factors play critical roles in angiogenesis, such as FGF, PDGF, HGF, VEGF, etc. The expression of FGF-16, PDGFb, HGF, VEGF and HIF-1α showed a rapid increase on day one, reached peaks on day 7 and decreased to normal levels on day 10 post-PDL only (Figure 3, table 2). Topical application of RPM showed a significant inhibitory effect on these growth factors/cytokine post-PDL exposure (p<0.05, n=3) (Figure 3, table 2).
Fig. 3.
Topical application of RPM significantly suppressed PDL-induced mRNA levels of angiogenic factors. The relative mRNA levels of FGF-16, PDGFb, HGF, VEGF, HIF-1α, TEK, EPAS1 and TGFβ3 are plotted versus the days post-PDL exposure. The data are presented as “Mean ± S.D.” and show the fold changes of mRNA levels of target genes (y-axes) as compared to the normal controls which are set as 1 (dashed lines). “#” indicates statistical significance (P<0.05) in the PDL only group as compared to the PDL+RPM group.
Table 2.
Topical RPM Inhibited PDL-induced mRNA Levels of Some Examples of Angiogenic Genes
| Day | P value | FGF16 | PDGFb | HGF | VEGFa | HIF1a | TEK | EPAS1 | TGFb3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 7.952 | 19.147 | 6.371 | 1.178 | 10.759 | 24.339 | 6.919 | 5.115 |
| P | 0.021 | 0.002 | 0.033 | 0.370 | 0.010 | 0.001 | 0.028 | 0.051 | |
| P1 | 0.026 | 0.003 | 0.025 | N.A. | 0.019 | 0.000 | 0.047 | N.A. | |
| P2 | 0.076 | 0.019 | 0.790 | N.A. | 0.068 | 0.059 | 0.066 | N.A. | |
| 3 | F | 0.449 | 227.458 | 5.197 | 20.783 | 5.667 | 26.554 | 3.531 | 49.670 |
| P | 0.658 | 0.000 | 0.049 | 0.002 | 0.041 | 0.001 | 0.097 | 0.000 | |
| P1 | N.A. | 0.000 | 0.065 | 0.009 | 0.069 | 0.006 | N.A. | 0.004 | |
| P2 | N.A. | 0.000 | 0.127 | 0.010 | 0.083 | 0.006 | N.A. | 0.001 | |
| 5 | F | 416.923 | 480.432 | 35.208 | 268.954 | 845.395 | 0.333 | 333.033 | 1727.746 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.729 | 0.000 | 0.000 | |
| P1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | N.A. | 0.000 | 0.000 | |
| P2 | 0.000 | 0.000 | 0.004 | 0.001 | 0.000 | N.A. | 0.115 | 0.000 | |
| 7 | F | 16.304 | 7.480 | 14.004 | 12.931 | 25.968 | 2.306 | 6.841 | 7.357 |
| P | 0.004 | 0.023 | 0.005 | 0.007 | 0.001 | 0.181 | 0.028 | 0.024 | |
| P1 | 0.000 | 0.036 | 0.007 | 0.001 | 0.001 | N.A. | 0.034 | 0.136 | |
| P2 | 0.000 | 0.070 | 0.026 | 0.034 | 0.009 | N.A. | 0.081 | 0.153 | |
| 10 | F | 1.435 | 5.732 | 2.260 | 0.249 | 0.396 | 1.072 | 1.364 | 1.957 |
| P | 0.309 | 0.041 | 0.186 | 0.787 | 0.689 | 0.400 | 0.325 | 0.222 | |
| P1 | N.A. | 0.123 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | |
| P2 | N.A. | 0.055 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | |
| 14 | F | 0.684 | 2.862 | 1.128 | 1.025 | 16.724 | 15.129 | 4.718 | 47.110 |
| P | 0.540 | 0.134 | 0.384 | 0.414 | 0.004 | 0.005 | 0.059 | 0.000 | |
| P1 | N.A. | N.A. | N.A. | N.A. | 0.683 | 0.452 | N.A. | 0.264 | |
| P2 | N.A. | N.A. | N.A. | N.A. | 0.013 | 0.021 | N.A. | 0.003 |
The fold changes of mRNA levels of each molecule are obtained by comparison to the control values which are set as 1. F and P are the values from ANOVA among the groups. Additional paired t-tests between the groups were performed only for those groups showing a statistical significance (p<0.05) by ANOVA. P1: Laser versus control; P2: Laser versus laser+RPM. N=3 or 4 animals for each treatment time point. N.A.: not available; p values from paired t-tests between two groups were not performed due to no statistical significance as determined by ANOVA.
Topical RPM had little effect on the increased mRNA levels of some angiogenic genes after PDL exposure, such as tyrosine endothelial kinase (TEK), endothelial PAS domain protein 1 (EPAS1) and transforming growth factor beta 3 (TGFβ3). The mRNA levels of TGFβ3 remained constantly increased by a factor of 2.0 as compared to the control throughout days 1 to 10 post-PDL exposure. Topical RPM showed inhibition on day 3, but no inhibitory effect during the rest of the study period (Figure 3, table 2).
More interestingly, topical RPM was able to facilitate the expression of many angiogenic genes after 10 days post-PDL treatment. Some examples were HIF-1α, TEK and TGFβ3. On day 14 post-PDL treatment, the mRNA levels of these genes showed a significant increase in the PDL+RPM group as compared to the PDL only group which remained at baseline (p<0.05, n=3, table 2), suggesting topical RPM was able to have a reversible effect on the expression of some angiogenic factors during the later stages of angiogenesis (Figure 3, table 2).
Topical RPM Effectively Penetrated the Stratum Corneum and Inhibited PDL-Induced Activation of P70S6K
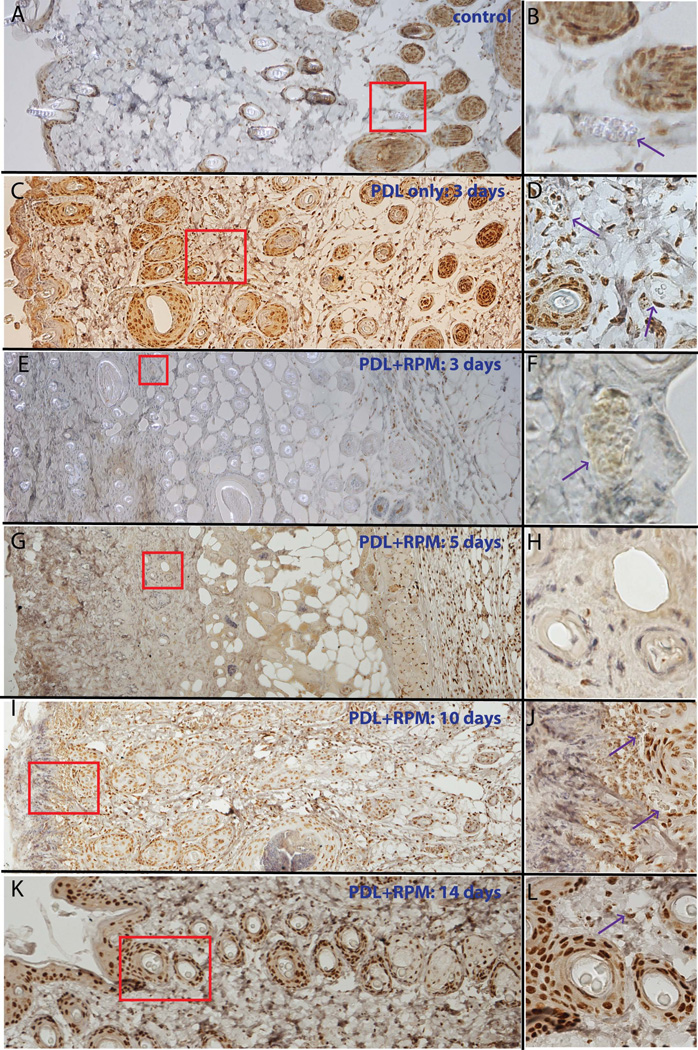
The pP70S6K showed some basal immunoreactive signals in the epidermis and hair follicles in normal control skin (Figure 4). The number of immunoreactive cells, particularly in photocoagulated blood vessels, increased in the dermis post-PDL (Figure 4). Application of 1% topical RPM for three to five consecutive days completely abolished pP70S6K immunoreactive signals from cells in the epidermis and dermis. Cells showing intact pP70S6K immunoreactive signals were approximately 768.4 µm from the skin surface (Figure 4, n=24 slides from 12 animals), which we estimated as the effective penetration depth of topical RPM in rodent skin. The pP70S6K immunoreactive signals were partially restored by day 10, and almost completely restored by day 14, in the epidermis and dermis (Figure 4).
Fig. 4.
Topical application of RPM significantly blocked PDL-induced activation of P70S6K. A, B. Epidermis and hair follicles show basal activity of P70S6K. C, D. The activity of P70S6K is elevated in the photocoagulated blood vessels (indicated by the arrows) in the dermis 3 days post-laser exposure. E, F, G, H. The activity of P70S6K in the epidermis and dermis, but not in muscular layers, is blocked with administration of 1% topical RPM for 3 (E, F) or 5 days (G, H). I, J. The activity of P70S6K is partially restored in the dermis, but not yet in the epidermis, 10 days post-laser exposure. Note the weaker intensity of pP70S6K immunoreactive signals of the hair follicles in (J) as compared to the basal activity of P70S6K in (B). K, L. The pP70S6K expression pattern in both epidermis and dermis returned to normal 14 days post-PDL exposure. The figures in the right column are a higher magnification of the boxed areas in the corresponding left panels. Arrows indicate blood vessels.
DISCUSSION
In this study, we investigated the molecular pathophysiological processes of PDL-induced angiogenesis in rodent skin and showed that topical application of 1% RPM could systematically suppress PDL-induced expression of many angiogneic genes with the most profound inhibitory effects in the early stages after light exposure, thus providing essential information on dosage and duration of topical RPM. Secondly, we showed that after topical application, RPM penetrated to an approximate depth of 768.4 µm into rodent skin.
HIF-1α is known to control the expression of hundreds of genes involved in inflammation, bioenergetics, proliferation, motility and apoptosis, and is considered as the master gene in control of angiogenesis [27,28]. The local skin hypoxia induced by laser exposure triggers angiogenesis. As a result, HIF-1α is translocated into nuclei and activates expressions of many angiogenic growth factors, including VEGF, FGF, PDGFb, etc. These growth factors subsequently activate their specific tyrosine kinase receptors and ultimately lead to angiogenesis. In this study, VEGF, FGF and PDGFb showed similar expression patterns to HIF-1α. All of these growth factors are downstream target genes of HIF-1α and participate in angiogenesis by acting as secondary order molecules to relay, activate and amplify the downstream transduction signals.
PDL-induced blood vessel injury not only activates angiogenesis but also other biological responses such as inflammation. In this study, we observed that many chemokines and pro-inflammatory cytokines, such as TGFβ3, showed increased mRNA levels, suggesting inflammatory / immunomodulatory responses are very active in skin post-PDL exposure. These cytokines not only control inflammatory responses but also regulate angiogenic pathways. For example, the expression of VEGF can be regulated by a TGFβ induced pathway which is HIF-1α independent [29]. Therefore, we concluded that increased mRNA levels of those angiogenic genes were the results from not only activation of angiogenesis pathways but also other intermingled biological processes, such as wound healing, defense and inflammatory responses.
In this study, we chose RPM to inhibit regeneration and revascularization of blood vessels post-PDL. Our data confirmed that topical RPM does effectively penetrate into rodent skin and induce an inhibitory effect on angiogenesis pathways: (1) the phosphorylation of pP70S6K in the epidermis and dermis was completely suppressed by topical application of RPM but not in deeper layers, which indicates the inhibition on mTOR/P70S6K executed at the sites of action by RPM is complete and sufficient; and (2) the expression profiles of the majority of angiogenic molecules we evaluated in this study showed a systematic and profound inhibition by topical RPM during the first seven days post-PDL exposure. However, to date, the intradermal concentration of RPM after topical application has yet to be determined. Therefore, determination of the intradermal concentration of RPM after topical application at the drug action sites in animals and human PWS subjects will be the focus of a future study. Nevertheless, the topical formulation of RPM developed in this study is effective in an animal model and can be used as a prototype that can be enhanced and adapted for human use in PWS patients.
We have demonstrated the profound inhibitory effects on angiogenesis pathways exhibited by topical RPM during the first seven days post-PDL exposure. However, the long-term effect on a photocoagulated blood vessel remains incompletely understood. Indeed, we found RPM showed an increase in the overall expression patterns of some angiogenic factors on day 14 post-PDL exposure, suggesting other signaling pathways rather than AKT/mTOR/P70S6K might have been activated. One possible candidate is the TGFβ signaling pathway. Our data shows the TGFβ signaling pathway is activated consistently throughout the entire process of angiogenesis and is not inhibited by RPM from the day 5 post-PDL exposure. The exact roles of the TGFβ signaling pathway on PDL-induced angiogenesis are unknown and will be the focus of future studies.
In conclusion, we have revealed the molecular pathophysiological processes of PDL-induced angiogenesis in rodent skin by systematically determining expression patterns of 86 angiogenic genes. Topical RPM can systematically suppress the PDL-induced early stage of angiogenesis via inhibition of the AKT/mTOR/P70S6K pathway. We have also shown the effective penetration depth of topical RPM in rodent skin. Our data have provided essential information on dosage, duration and molecular mechanisms for potential clinical applications of topical RPM for PWS treatment.
Acknowledgments
We greatly appreciate the assistance of Amanda Dickson of the Sue & Bill Gross Stem Cell Research Center at the University of California, Irvine, for her expertise on the real time PCR and histology image acquisition process experiments. We are also grateful to Linda Li at the Beckman Laser Institute and Medical Clinic for help in biopsy tissue processing and immunohistochemistry. Institutional support was provided by the Arnold and Mabel Beckman Foundation and the David and Lucile Packard Foundation.
Funding/Support: This study was supported in part by NIH AR063766 to WT, AR47551 and AR59244 to JSN, and the American Society for Laser Medicine and Surgery research grant F03.12 and F01.13 to WT.
Footnotes
Financial Disclosure: None declared.
DISCLAIMER Any views expressed here represent personal opinion and do not necessarily reflect those of the U.S. Department of Health and Human Services or the United States federal government.
REFERENCES
- 1.Mulliken JB, Young AR. Vascular Birthmarks--Hemangiomas and Malformations. Philadelphia, PA: W.B. Saunders Co.; 1988. [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–222. [PubMed] [Google Scholar]
- 3.Pratt AG. Birthmarks in infants. Arch Dermatol Syphilol. 1953;67:302–305. doi: 10.1001/archderm.1953.01540030065006. [DOI] [PubMed] [Google Scholar]
- 4.Milliken JB, Young AR. Vascular Birthmarks: Hemangiomas and Malformations. Philadelphia, PA: W.B. Saunders Co.; 1988. [Google Scholar]
- 5.Dohil MA, Baugh WP, Eichenfield LF. Vascular and pigmented birthmarks. Pediatr Clin Noth Am. 2000;47:783–812. doi: 10.1016/s0031-3955(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 6.Pratt AG. Birthmarks in infants. Arch Dermatol. 1967;67:302–311. doi: 10.1001/archderm.1953.01540030065006. [DOI] [PubMed] [Google Scholar]
- 7.Heller A, Rafman S, Zvagulis I, Pless IB. Birth-defects and psychosocial adjustment. Am J Dis Child. 1985;139:257–263. doi: 10.1001/archpedi.1985.02140050051021. [DOI] [PubMed] [Google Scholar]
- 8.Malm M, Carlberg M. Port-wine stain - a surgical and psychological problem. Ann Plast Surg. 1988;20:512–516. doi: 10.1097/00000637-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kalick SM. Toward an interdisciplinary psychology of appearances. Psychiatry. 1978;41:243–253. [PubMed] [Google Scholar]
- 10.Lever WF, Schaumburg-Lever G. Histopathology of the Skin. 7th ed. Philadelphia, PA: J.B. Lippincott Co.; 1990. [Google Scholar]
- 11.Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol. 1991;17:76–79. doi: 10.1111/j.1524-4725.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 12.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-Weber Syndrome and Port-Wine Stains Caused by Somatic Mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan W, Chernova M, Gao L, Sun V, Liu H, Jia W, et al. Sustained activation of c-Jun N-terminal and extracellular signal-regulated kinases in port-wine stain blood vessels. J Am Acad Dermatol. 2014 doi: 10.1016/j.jaad.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 15.Nelson JS, Milner TE, Anvari B, Tanenbaum BS, Kimel S, Svaasand LO, et al. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch Dermatol. 1995;131:695–700. [PubMed] [Google Scholar]
- 16.Katugampola GA, Lanigan SW. Five years' experience of treating port wine stains with the flashlamp-pumped pulsed dye laser. Br J Dermatol. 1997;137:750–754. [PubMed] [Google Scholar]
- 17.van der Horst CM, Koster PH, de Borgie CA, Bossuyt PM, van Gemert MJ. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338:1028–1033. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 18.Yohn JJ, Huff JC, Aeling JL, Walsh P, Morelli JG. Lesion size is a factor for determining the rate of port-wine stain clearing following pulsed dye laser treatment in adults. Cutis. 1997;59:267–270. [PubMed] [Google Scholar]
- 19.Tan W, Jia W, Sun V, Mihm MC, Jr, Nelson JS. Topical rapamycin suppresses the angiogenesis pathways induced by pulsed dye laser: molecular mechanisms of inhibition of regeneration and revascularization of photocoagulated cutaneous blood vessels. Lasers Surg Med. 2012;44:796–804. doi: 10.1002/lsm.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest Ophthalmol Vis Sci. 2005;46:454–460. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Updat. 2001;4:378–391. doi: 10.1054/drup.2002.0227. [DOI] [PubMed] [Google Scholar]
- 24.Jia W, Sun V, Tran N, Choi B, Liu SW, Mihm MC, Jr, et al. Long-term blood vessel removal with combined laser and topical rapamycin antiangiogenic therapy: implications for effective port wine stain treatment. Lasers Surg Med. 2010;42:105–112. doi: 10.1002/lsm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loewe R, Oble DA, Valero T, Zukerberg L, Mihm MC, Jr, Nelson JS. Stem cell marker upregulation in normal cutaneous vessels following pulsed-dye laser exposure and its abrogation by concurrent rapamycin administration: implications for treatment of port-wine stain birthmarks. J Cutan Pathol. 2010;37(Suppl 1):76–82. doi: 10.1111/j.1600-0560.2010.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson JS, Jia W, Phung TL, Mihm MC., Jr Observations on enhanced port wine stain blanching induced by combined pulsed dye laser and rapamycin administration. Lasers Surg Med. 2011;43:939–942. doi: 10.1002/lsm.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekiguchi Y, Zhang J, Patterson S, Liu L, Hamada C, Tomino Y, et al. Rapamycin inhibits transforming growth factor beta-induced peritoneal angiogenesis by blocking the secondary hypoxic response. J Cell Mol Med. 2012;16:1934–1945. doi: 10.1111/j.1582-4934.2011.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]