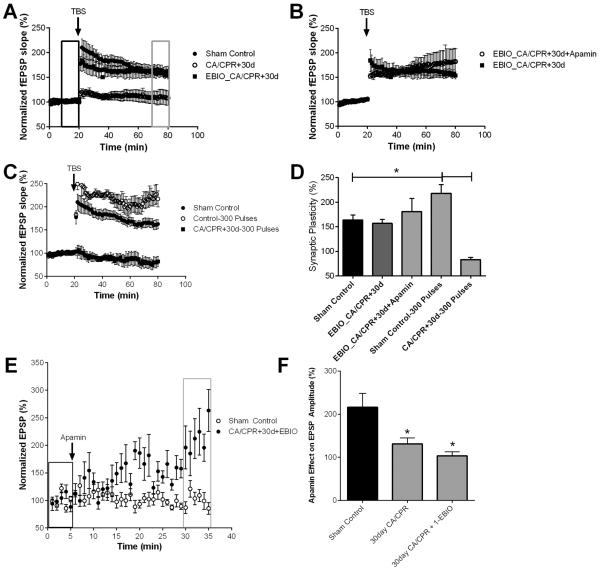
Figure 7.
1EBIO prevents CA/CPR loss of SK2 channel activity in synaptic plasticity. A) Time course of fEPSP slope (mean ± SEM) from sham (solid circles) and mice 30 days (open circles) after cardiac arrest and cardiopulmonary resuscitation (CA/CPR) and mice treated with 1-EBIO early after CA/CPR and time course recorded 30 days later (solid square). Arrow indicates timing of theta burst stimulus (TBS; 40 pulses). B) Time course of fEPSP slope (mean ± SEM) from 1-EBIO treated mice 30 days after CA/CPR in the presence (open square) and absence (solid square) of 100 nM apamin. Arrow indicates timing of theta burst stimuls (TBS; 40 pulses) C) Time course of fEPSP slope (mean ± SEM) from sham control mice stimulated with standard 40 pulse TBS (solid circle) or 300 pulse TBS stimulus (open square) and mice 30 days after CA/CPR stimulated with 300 pulse TBS. Arrow indicates timing of theta burst stimulus (TBS; 40 or 300 pulses) D) Quantification of change in fEPSP slope following TBS. Average fEPSP slope (mean ± SEM) 60 minutes after TBS (in grey box in A normalized to baseline (black box in A). * P < 0.05. E) Time course of EPSP amplitude (mean ± SEM) from sham control mice and 30 days after CA/CPR mice treated with 1-EBIO before (black rectangle) and after (grey rectangle) a 30 minute application of 100 nM apamin. F) Quantification of the effect of apamin on EPSP amplitude. EPSP amplitude 30 minutes after application of apamin (grey square in E) normalized to baseline (black square in E).