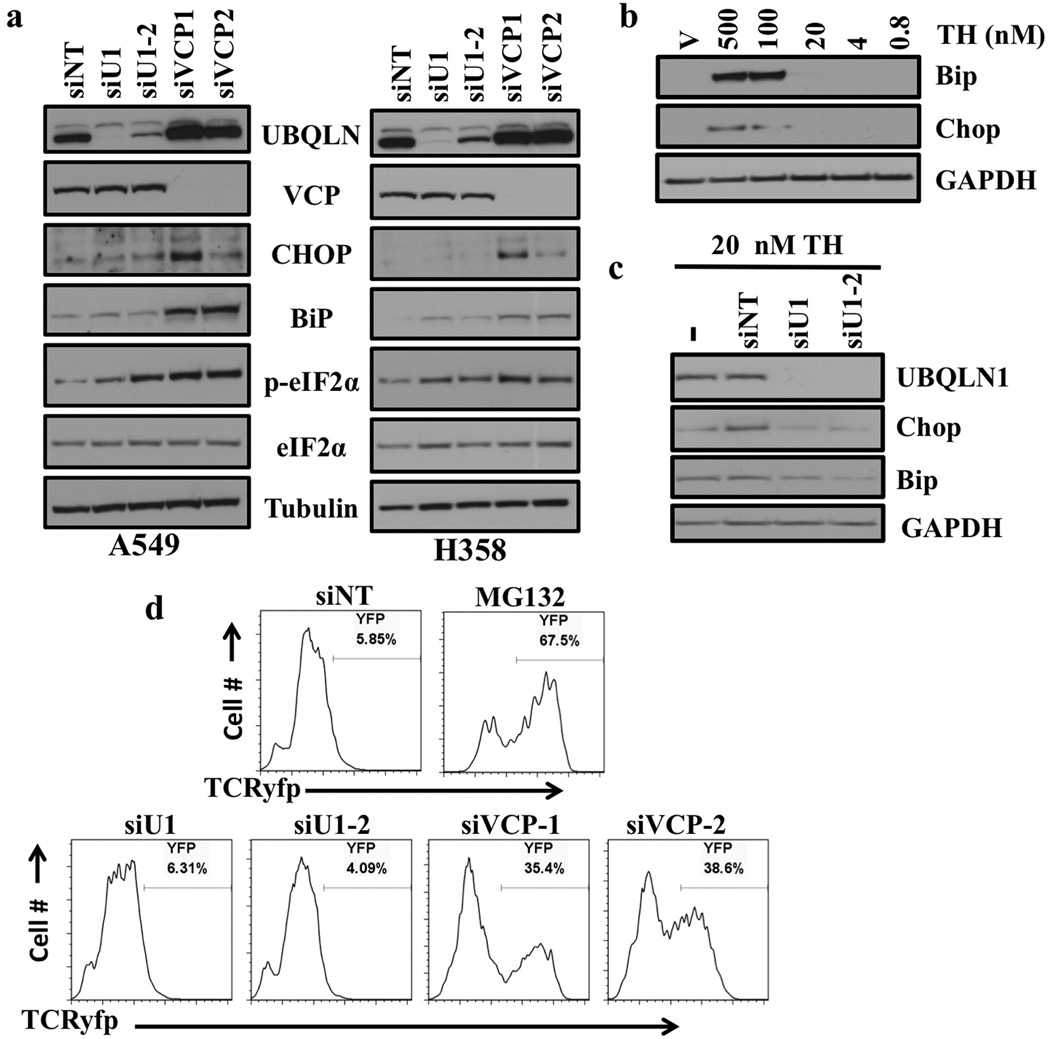
Figure 1. Loss of UBQLN1 does not induce ER stress or alter ERAD.
(a) Western blot analysis of proteins involved in unfolded protein response and ER stress response. Cells were transfected with either non targeting (siNT) siRNA or two different siRNAs targeting UBQLN1 (siU1 and siU2) and two different siRNAs targeting VCP (siVCP1 and siVCP2). After 72 hrs of transfection, cells were harvested and analyzed for protein expression. (b) Western Blot analysis of proteins involved in ER stress response. A549 cells were treated with vehicle or with indicated concentrations of Thapsigargin (TH). (c) Western Blot analysis of proteins involved in ER stress response. A549 cells were transfected with either non targeting (siNT) siRNA or two different siRNAs targeting UBQLN1 (siU1 and siU2). 48 hrs post siRNA transfections cells were treated with vehicle or with 20nM of Thapsigargin (TH) for 30 min and harvested 24 hrs later. (d) UBQLN1 loss does not alter ERAD. 293T cells stably expressing TCRyfp, a well-known ERAD substrate, were untreated (NT), treated with MG132, or transfected with the indicated siRNA. Cells were subjected to flow cytometry 24 hrs after MG132 treatment or 72 hrs post siRNA transfection to determine the intensity of yfp signal. MG132 treatment is used as a positive control, as ERAD is known to rely on the proteasome for destruction of substrates.