Abstract
AIM: The expression pattern of endoglin (CD105) in hepatocellular carcinoma (HCC) has not been reported so far. We hypothesized that CD105 could differentially highlight a subset of microvessels in HCC, and intratumoral microvessel density (IMVD) by CD105 immunostaining (IMVD-CD105) could provide better prognostic information than IMVD by CD34 immunostaining (IMVD-CD34).
METHODS: Paraffin blocks of tumor and adjacent non-tumorous liver tissues from 86 patients who underwent curative resection of HCC were used for this study. Serial sections were stained for CD105 and CD34, respectively, to highlight the microvessels. IMVD was counted according to a standard protocol.
RESULTS: In the HCC tissues, CD105 was either negatively or positively stained only in a subset of microvessels. In contrast, CD34 showed positive and more extensive microvessel staining in all cases examined. However, in the adjacent non-tumorous liver sections, CD105 showed a diffuse pattern of microvessel staining in 20 of 86 cases, while CD34 showed negative or only focal staining of the sinusoids around portal area. Correlation with clinicopathological data demonstrated that lower scores of IMVD-CD105 were found in larger sized tumors [mean 41.4/0.74 mm2 (>5 cm tumor) vs 65.9/0.74 mm2 (≤5 cm tumor), P = 0.043] and more aggressive tumors, as indicated by venous infiltration [36.8/0.74 mm2 (present) vs 64.2/0.74 mm2 (absent), P = 0.020], microsatellite nodules [35.1/0.74 mm2 (present) vs 65.9/0.74 mm2 (absent), P = 0.012], and advanced TNM tumor stage [38.8/0.74 mm2 (stage 3 or 4) vs 68.3/0.74 mm2 (stage 1 or 2), P = 0.014]. No prognostic significance was observed when median values were used as cut-off points using either IMVD-CD105 or IMVD-CD34. However, the presence of the diffuse pattern of CD105 expression in the adjacent non-tumorous liver tissues predicted a poorer disease-free survival (median 8.6 vs 21.5 mo, P = 0.026).
CONCLUSION: Our data demonstrate that a lower IMVD-CD105 is associated with larger and more aggressive tumors. In this study, IMVD-CD105 did not provide significant prognostic information. However, active angiogenesis as highlighted by diffuse CD105 staining of the microvessels in the adjacent non-tumorous liver tissues is predictive of early recurrence.
Keywords: Hepatocellular carcinoma, Endoglin, Intratumoral microvessel density
INTRODUCTION
Angiogenesis, the formation of new capillaries from a pre-existing vasculature, is implicated in tumor development, growth, progression, and metastasis[1-3]. Different from that in the normal physiological condition, the fine balance of modulation between proangiogenic and antiangiogenic factors is disturbed in tumor microenvironment, thus leading to abnormal vessel growth. It is known that tumor-associated endothelial cells in the neovasculature proliferate 20 to 2000 times more rapidly than those found in the normal vasculature[4,5]. The characteristics of microvessels in tumors are different from that developed under normal physiological conditions[6]. In fact, intratumoral microvessel density (IMVD) has been extensively investigated and was found to be a useful prognostic marker in many types of cancers[7].
Hepatocellular carcinoma (HCC) is a highly vascularized tumor on angiography. Its neovascularization is featured by sinusoidal capillarization[8]. However, not much is known about the exact mechanism and contribution of angiogenesis in different stages of HCC development. Several studies have shown that certain endothelial markers, in particular CD34, are expressed diffusely in the microvessels of HCC, and that their levels of expression correlate with prognosis of the patients[9-11]. A previous study by our group showed that IMVD assessed by CD34 immunostaining (IMVD-CD34) was a good prognostic marker of recurrence after hepatectomy in patients whose tumors were less than or equal to 5 cm in diameter[11]. However, CD34 is a pan-endothelial cell marker that reacts not only with proliferating vessels but also with established vessels in the tumor. Therefore, CD34 might not be an ideal marker of tumor neovascularization in HCC.
Endoglin (CD105) is a homodimeric membrane glycoprotein expressed on endothelial cells that can bind to transforming growth factor-β1 and transforming growth factor-β3[12]. CD105 is only weakly expressed in normal tissues, but it is strongly expressed in tumor endothelia[13-18]. Recent studies have suggested that CD105 is a proliferation-associated marker of endothelial cells[13], and that its expression correlates strongly with cell proliferation markers in tumor endothelia[12]. Moreover, CD105 has been demonstrated to be a good tumor angiogenesis marker in breast cancer[19], brain tumor[20], malignant melanoma[21] and colorectal carcinoma[22]. Detection of CD105 in endothelial cells by immunohistochemical staining has been reported to provide a superior angiogenesis marker compared with the conventional CD34 staining in non-small cell lung cancer[18] and multiple myeloma[23]. Evaluation of tumor tissue expression of CD105 in correlation with clinicopathological parameters has revealed its diagnostic and prognostic significance in cancers such as breast cancer[24,25], squamous cell carcinoma of the oral cavity[26], prostate cancer[27], and renal cell carcinoma[28].
To date, not much is known about the exact mechanism of tumor angiogenesis in HCC, and the expression pattern of CD105 in HCC has not been reported before. Supported by the evidence shown in other tumors, we hypothesized that CD105 might be able to differentially highlight a subset of newly sprouted and immature microvessels in HCC, and tumor IMVD by CD105 immunostaining (IMVD-CD105) might provide a more informative angiogenesis score than IMVD-CD34, especially in terms of correlation with clinicopathological parameters.
MATERIALS AND METHODS
Patients and tissue samples
Eighty-six patients (67 men, 19 women) who underwent curative resection of HCC, defined as macroscopically complete removal of the tumor, in the Department of Surgery of the University of Hong Kong at Queen Mary Hospital were studied. None of the patients had received any preoperative treatment such as transarterial chemoembolization, and no postoperative adjuvant therapy was given. The study was approved by the Institutional Review Board of our institution.
The mean±SD maximum diameter of the tumors was 8.0 +/- 5.0 cm (range 1.5 cm to 22.0 cm). Thirty-three patients (38%) had tumor ≤5 cm in diameter. Tissue specimens were taken from the tumors and from adjacent non-tumorous livers. Fresh tissue specimens were fixed in 10% buffered formalin and embedded in paraffin, and 4-μm-thick sections were prepared for immunohistochemical study.
Immunohistochemical staining for CD34 and CD105
Tumorous and non-tumorous sections were immunostained with human CD34 monoclonal antibody (1:100; QBEND-10, Dako, Carpenteria, CA, USA) and CD105 monoclonal antibody (1:1000; SN6H, Dako, Carpenteria, CA, USA). Consecutive paraffin sections were used for the staining of CD34 and CD105. Vascularization was demonstrated by an immunohistochemical analysis for CD105 using a catalyzed signal amplification system (Dako), based on the peroxidase-catalyzed deposition of a biotinylated phenol compound tyramide, while LSAB kit from Dako was used for CD34 staining. The negative controls were obtained by substituting the primary antibodies with mouse immunoglobulin G. IMVD was evaluated according to Gasparini’s criteria[29] by two independent observers as described in a previous report[11]. The mean microvessel count of the five most vascular areas was taken as the MVD, which was expressed as the absolute number of microvessels per 0.74 mm2 (×200 field).
Clinicopathological and follow-up data
All clinicopathological data were collected prospectively in a computerized database, and all patients were followed and monitored regularly for tumor recurrence by serum alpha fetoprotein (AFP) level monthly and chest X-ray together with computed tomography (CT) scan every 3 mo. The median follow-up time of all patients was 36 mo (range 20 to 50 mo). A diagnosis of recurrence was based on typical imaging appearance in CT scan and an elevated AFP level, and if necessary, fine needle aspiration cytology.
Statistical analysis
Student t-test was used for comparison of continuous variables between groups. The correlation between continuous variables was performed using the Spearman rank correlation test. Cumulative disease-free survival was computed using the Kaplan-Meier method and comparison between groups was done by the log-rank test.
Clinicopathological variables that were correlated with IMVD included gender, age (≤ or >60 years), hepatitis B surface antigen (HBsAg) status, presence or absence of cirrhosis in the non-tumorous livers, tumor size (≤ or >5 cm), tumor differentiation by Edmonson grade (1-2 or 3-4), any tumor encapsulation, venous invasion, microsatellite nodules, and pTNM stage (I/II or III/IVA). All statistical analyses were performed using the SPSS statistical software (SPSS/PC+, SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
CD105 and CD34 staining in tumor and adjacent non-tumorous liver
Figure 1 shows the typical staining patterns of CD105 immunostaining in the tumor and the adjacent non-tumorous sections of the liver. The mean score of IMVD-CD105 was 50.8±54.8/0.74 mm2 (range 0-240.0), whereas the mean score of IMVD-CD34 was 125.6±53.2/0.74 mm2 (range 28.2-283.4) (P < 0.001). There were no significant differences between IMVD values obtained by the two independent scorers.
Figure 1.
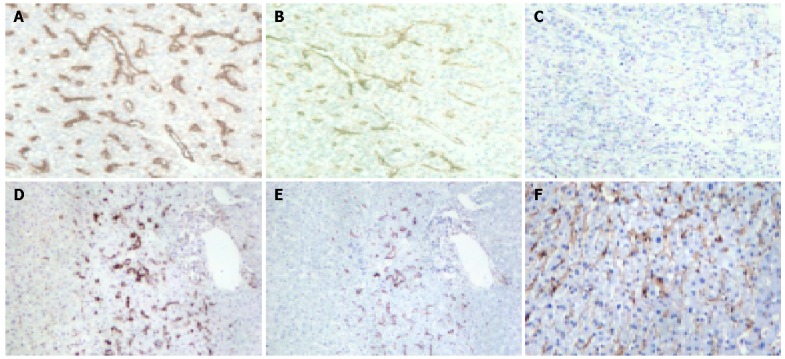
Expression of CD105 and CD34 as shown by the brown staining of the vasculature in tumor and adjacent non-tumorous liver (×100). A-C are serial sections of tumor tissue, where A shows the CD34 expression, B shows the CD105 expression, and C shows the negative control. D and E are serial sections of adjacent non-tumorous liver, where D shows the CD34 expression, and E shows the CD105 expression. Both D and E illustrate typical focal staining of vessels around the portal vein. F shows a case of diffuse CD105 staining in the non-tumorous liver, whereas such pattern of staining was not seen in the non-tumorous liver by CD34 staining.
Results from this study of 86 HCC cases revealed some similarities but also some differences between CD34 and CD105 staining. Similar to the CD34 staining observed in the present study, and that from others reported in HCC[9-11,30], highlighted microvessels by CD105 also showed three patterns of expression on the tumor tissue sections: sinusoid-like, branching, and small without apparent lumina (endothelial sprouts). Distinctively, the expression pattern of CD105 in HCC differed from that of CD34 in the following aspects. First, when consecutive slides of tumor tissue sections were compared, the number of vessels highlighted by CD105 was considerably lower than that by CD34, although a statistically significant positive correlation was observed between the MVD scores by the two markers (Figure 2). Second, in all tumor sections examined, CD105 was completely negative in 28/86 cases, while CD34 was positive in all of the cases examined. Third, in non-tumorous tissues, CD105 showed variable patterns of staining, including a diffuse pattern of staining in some cases, whereas CD34 was uniformly negative in non-tumorous liver except for sparse sinusoid staining distributed focally around the portal tract.
Figure 2.
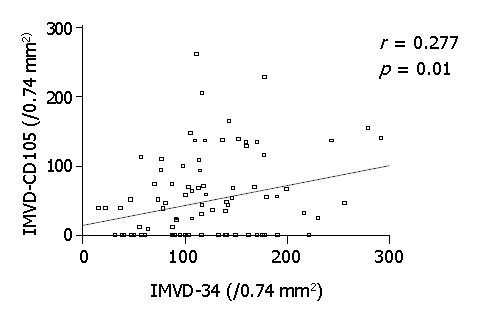
Graph of scatter plots showing correlation between scores of IMVD-CD105 vs IMVD-CD34.
Correlation between IMVD and clinicopathological parameters
Table 1 summarizes the analysis of IMVD-CD105 and IMVD-CD34 in relation to various clinicopathological parameters. No significant differences were found between either IMVD-CD105 or IMVD-CD34 and gender, age or hepatitis B infection status of the patients. However, IMVD-CD105 and IMVD-CD34 were significantly related to several pathological variables. Both IMVD-CD105 and IMVD-CD34 were significantly lower in tumors > 5 cm compared to tumors ≤5 cm in diameter (P = 0.043 and 0.002, respectively). Furthermore, both showed a significant correlation with pTNM staging, and a lower IMVD observed in tumors with more advanced staging (P = 0.014 and 0.005, respectively). However, only lower scores of IMVD-CD105 showed a statistically significant association with venous invasion [36.8/0.74 mm2 (present) vs 64.2/0.74 mm2 (absent), P = 0.02], and microsatellite nodules [35.1/0.74 mm2 (present) vs 65.9/0.74 mm2 (absent), P = 0.012].
Table 1.
Correlation of IMVD-CD105 and IMVD-CD34 with clinicopathological parameters.
| Clinical parameters |
IMVD-CD105 (/0.74 mm2) |
IMVD-CD34 (/0.74 mm2) |
||||||
| n | mean | SD | P | n | mean | SD | P | |
| Gender | ||||||||
| Male | 67 | 49.29 | 55.17 | 0.631 | 67 | 124.9 | 53.51 | 0.823 |
| Female | 19 | 56.19 | 54.4 | 19 | 128.1 | 53.54 | ||
| Age (yr) | ||||||||
| ≤60 years | 58 | 43.96 | 48.21 | 0.095 | 58 | 121.1 | 58.19 | 0.257 |
| >60 years | 28 | 65.01 | 64.99 | 28 | 135 | 40.39 | ||
| HBsAg | ||||||||
| Positive | 69 | 46.35 | 49.36 | 0.121 | 69 | 121.5 | 53.02 | 0.227 |
| Negative | 17 | 70.08 | 73.87 | 17 | 139.4 | 52.48 | ||
| Histopathological parameters | ||||||||
| Tumor size | ||||||||
| ≤ 5 cm | 33 | 65.9 | 58.98 | 0.043 | 33 | 147.8 | 53.41 | 0.002 |
| >5 cm | 53 | 41.42 | 50.26 | 53 | 111.9 | 48.68 | ||
| Tumor encapsulation | ||||||||
| Absent | 53 | 48.31 | 48 | 0.497 | 53 | 120.3 | 54.19 | 0.193 |
| Present | 33 | 56.72 | 65.04 | 33 | 135.9 | 52.01 | ||
| Venous invasion | ||||||||
| Absent | 44 | 64.17 | 60.96 | 0.02 | 44 | 133.6 | 50.14 | 0.157 |
| Present | 42 | 36.83 | 43.91 | 42 | 117.3 | 55.64 | ||
| Microsatellite lesions | ||||||||
| Absent | 48 | 65.88 | 62.15 | 0.012 | 48 | 129.6 | 52.55 | 0.401 |
| Present | 38 | 35.06 | 41.02 | 38 | 119.7 | 52.53 | ||
| Adjacent non-tumorous liver | ||||||||
| Normal (0) | 6 | 59.26 | 52.46 | 0.602 (0&1) | 6 | 103 | 40.09 | 0.602 (0&1) |
| Chronic hepatitis (1) | 38 | 43.14 | 57.47 | 0.817 (0&2) | 38 | 118.4 | 47.69 | 0.118 (1&2) |
| Cirrhosis (2) | 42 | 53.84 | 53.49 | 0.537 (1&2) | 42 | 135.4 | 58.31 | 0.158 (0&2) |
| Edmonson grade | ||||||||
| 1 & 2 | 21 | 83.73 | 65.87 | 0.079 | 21 | 162.9 | 41.95 | 0.013 |
| ≥3 | 65 | 48.47 | 54.21 | 65 | 117.3 | 51.12 | ||
| TNM stage | ||||||||
| I & II | 35 | 68.26 | 63.67 | 0.014 | 35 | 144.9 | 55.42 | 0.005 |
| III & IVA | 51 | 38.84 | 44.52 | 51 | 112.4 | 47.84 | ||
HBsAg, hepatitis B surface antigen; TNM, tumor-node-metastasis.
Disease-free survival analysis
Disease-free survival analysis was performed in 81 patients after 5 patients with hospital mortality or palliative resection (i.e., positive resection margin) were excluded. No prognostic significance was observed when median values were used as cut-off points using either IMVD-CD105 or IMVD-CD34. However, as shown in Figure 3, when the patients were segregated by the presence and absence of diffuse CD105 staining patterns in the adjacent non-tumorous liver tissues, the patients with diffuse CD105 staining in the adjacent non-tumorous livers had a much poorer prognosis than those patients without such a pattern of CD105 staining (median disease-free survival 8.6 vs 21.5 mo, P = 0.026).
Figure 3.
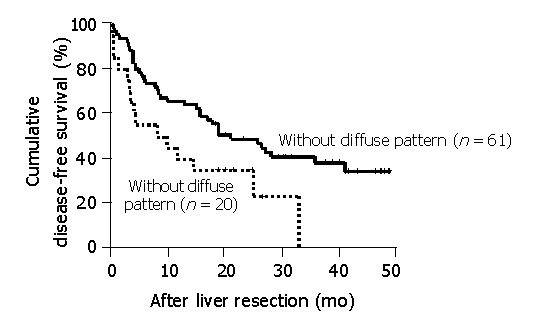
Disease-free survival analysis shows the differences in disease-free survival in patients with or without diffuse pattern of CD105 staining in non-tumorous livers. Five patients were excluded from the analysis because of hospital mortality or palliative resection with positive resection margins.
DISCUSSION
Previous reports have demonstrated that IMVD-CD34 is a better prognostic marker for HCC than IMVD assessed by many other endothelial markers, such as von Willebrand marker (vWF), CD31, and UEA-1[10,11,31]. However, CD34 is a pan-endothelial cell marker, therefore, it may not be able to reflect the exact angiogenesis activity in a tumor. According to some previous reports, CD105 is an endothelial marker that appears to react only with the endothelial cells in the newly formed vessels, and in particular, the immature tumor blood vessels[13-18]. Moreover, studies in other cancers have suggested that scores of IMVD-CD105 might be superior to those of IMVD-CD34 in terms of prognostic information[18,23]. To our knowledge, this is the first study that evaluated the clinicopathological significance of IMVD-CD105 in HCC, which is of particular interest because HCC is one of the most vascular solid tumors. Overall, in agreement with those reported for other cancers in the literature, our immunostaining confirmed that CD105 could stain a subset of microvessels and the staining patterns of CD105 in both tumors and non-tumorous liver differed from those stained by CD34 in certain aspects.
Correlation with clinicopathological parameters in this study demonstrated significantly lower IMVD scores in larger and more advanced stage tumors with both CD105 and CD34 as the endothelial markers. Moreover, lower scores of IMVD-CD105 were associated with more aggressive tumors as indicated by microscopic venous invasion and microsatellite nodules (Table 1). The latter correlations were probably indirect ones related to the higher frequencies of microscopic venous invasion and microsatellite nodules in larger tumors. The findings of a clinicopathological correlation with IMVD-CD105 were apparently contrary to those reported in other solid tumor types such as breast cancer and non-small cell lung cancer[18,24,25], in which higher rather than lower IMVD scores were associated with more aggressive tumors. However, as reviewed by Gasparini et al[32], most retrospective studies in breast cancer were on early-stage tumors, and hence angiogenesis appears to be a good prognostic marker only in early-stage group of breast cancer. Similarly, our previous study also illustrated that IMVD-CD34 was a good prognostic marker in small tumors ≤5 cm only[11]. The HCC cases studied by Tanigawa et al[10], who demonstrated a worse prognosis with a higher IMVD-CD34, were also mainly small tumors ≤5 cm in diameter. In this study, the majority of tumors were >5 cm in diameter, and both IMVD-CD105 and IMVD-CD34 decreased with increase in tumor size. In support of our data, the IMVD-CD34 analysis of 71 HCC cases by El-Assal et al[9] also found that the larger (>5 cm) tumors showed a trend to decrease IMVD. Neither IMVD-CD105 nor IMVD-CD34 showed a prognostic influence in this study of predominantly large HCCs.
Blood vessels could provide support for oxygen and nutrients, as well as many paracrine factors to its surrounding tumor cells[1,33,34]. Although it has been well accepted that tumor growth is dependent on angiogenesis[1], whether IMVD could truly reflect the angiogenic activity of tumors remains controversial. As pointed out by a recent review on IMVD from Folkman’s group[7], IMVD reflects intercapillary distance, and therefore it may reflect the metabolic need of its surrounding tumor cells. While our finding of a lower IMVD in larger tumors should not be interpreted as suggesting that a bigger size tumor is less angiogenesis dependent, it may, however, indicate that tumor cells in a bigger sized tumor adapt to survive with less metabolic demand. It has been reported that oxygen consumption was much less in tumor tissues as compared with normal counterparts[1,35]. Another plausible explanation is that the turnover of tumor cells is much shorter than that of endothelial cells, resulting in an increased intercapillary distance as the tumor size increases[36,37]. This is particularly interesting in the case of HCC, which could grow very much larger in size compared to other types of tumors, as indicated by a mean diameter of 8.0 cm in our group of patients. Our results seem to support the notion that as the tumor cells expand in HCC, they can become more and more accustomed to a lower metabolic demand with an increase in tumor size[35].
The adjacent non-tumorous livers were also included in this study. Interestingly, when we segregated the patients by CD105 stained vessel patterns in the adjacent non-tumorous livers according to the presence or absence of a well-diffuse pattern, the disease-free survival in those patients with a diffuse pattern of CD105 staining in the adjacent non-tumorous livers was statistically worse than that of patients without a diffuse pattern of CD105 expression in the adjacent non-tumorous livers. The finding that the diffuse pattern of CD105 staining in the adjacent non-tumorous liver can predict early recurrence is interesting but not surprising, because unlike other cancer types, the adjacent non-tumorous tissues in HCC patients were not normal in most cases. The liver of HCC patients is usually infected with chronic hepatitis B or C viruses, which lead to chronic hepatitis or even cirrhosis. Angiogenesis might have an important role in these disease processes too. Angiogenesis in the non-tumorous liver may enhance the growth of intrahepatic metastasis or development of multicentric tumors, both of which contribute to postoperative recurrence. Although the exact mechanism is unknown, our data suggest that the detection of the diffuse pattern of CD105 in the adjacent non-tumorous liver represents an unfavorable factor to those patients recovering from HCC resection. The relationship between angiogenesis in the non-tumorous liver and postoperative recurrence of HCC warrants further investigation.
In conclusion, contrary to those reported on other types of cancers where CD105 was found to be a good prognostic marker or even better than CD34, our data showed that IMVD-CD105 in HCC did not correlate with the prognosis of patients. However, our data illustrated that CD105 could stain a subset of microvessels differently from that stained by CD34 in HCC tissues. Our clinicopathological analysis demonstrated that lower scores of IMVD-CD105 were found in larger sized and more aggressive tumors. Furthermore, different from CD34, CD105 could also stain microvessels in the adjacent non-tumorous liver. The presence of well-diffuse patterns of CD105 expression in the adjacent non-tumorous liver could predict early disease recurrence. Further studies are merited to clarify the mechanisms involved in these associations.
Footnotes
Supported by the Sun C.Y. Research Foundation for Hepatobiliary and Pancreatic Surgery of The University of Hong Kong
Assistant Editor Guo SY Edited by Wang XL
References
- 1.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 4.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herlyn M. Endothelial cells as targets for tumor therapy. J Immunother. 1999;22:185. doi: 10.1097/00002371-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, Dvorak HF. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta. 1989;948:305–326. doi: 10.1016/0304-419x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 7.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 8.Kin M, Torimura T, Ueno T, Inuzuka S, Tanikawa K. Sinusoidal capillarization in small hepatocellular carcinoma. Pathol Int. 1994;44:771–778. doi: 10.1111/j.1440-1827.1994.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 9.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 10.Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology. 1997;26:1216–1223. doi: 10.1053/jhep.1997.v26.pm0009362365. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775–1785. doi: 10.1200/JCO.2002.07.089. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 13.Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, Letarte M, Vitetta ES, Thorpe PE. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1:1623–1634. [PubMed] [Google Scholar]
- 14.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–1044. [PubMed] [Google Scholar]
- 15.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 16.Westphal JR, Willems HW, Schalkwijk CJ, Ruiter DJ, de Waal RM. A new 180-kDa dermal endothelial cell activation antigen: in vitro and in situ characteristics. J Invest Dermatol. 1993;100:27–34. doi: 10.1111/1523-1747.ep12349946. [DOI] [PubMed] [Google Scholar]
- 17.Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin Cancer Res. 1999;5:371–382. [PubMed] [Google Scholar]
- 18.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H. Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7:3410–3415. [PubMed] [Google Scholar]
- 19.Bodey B, Bodey B, Siegel SE, Kaiser HE. Over-expression of endoglin (CD105): a marker of breast carcinoma-induced neo-vascularization. Anticancer Res. 1998;18:3621–3628. [PubMed] [Google Scholar]
- 20.Bodey B, Bodey B, Siegel SE, Kaiser HE. Upregulation of endoglin (CD105) expression during childhood brain tumor-related angiogenesis. Anti-angiogenic therapy. Anticancer Res. 1998;18:1485–1500. [PubMed] [Google Scholar]
- 21.Bodey B, Bodey B, Siegel SE, Kaiser HE. Immunocytochemical detection of endoglin is indicative of angiogenesis in malignant melanoma. Anticancer Res. 1998;18:2701–2710. [PubMed] [Google Scholar]
- 22.Akagi K, Ikeda Y, Sumiyoshi Y, Kimura Y, Kinoshita J, Miyazaki M, Abe T. Estimation of angiogenesis with anti-CD105 immunostaining in the process of colorectal cancer development. Surgery. 2002;131:S109–S113. doi: 10.1067/msy.2002.119361. [DOI] [PubMed] [Google Scholar]
- 23.Pruneri G, Ponzoni M, Ferreri AJ, Decarli N, Tresoldi M, Raggi F, Baldessari C, Freschi M, Baldini L, Goldaniga M, et al. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol. 2002;118:817–820. doi: 10.1046/j.1365-2141.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, Bundred N. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 25.Dales JP, Garcia S, Bonnier P, Duffaud F, Andrac-Meyer L, Ramuz O, Lavaut MN, Allasia C, Charpin C. CD105 expression is a marker of high metastatic risk and poor outcome in breast carcinomas. Correlations between immunohistochemical analysis and long-term follow-up in a series of 929 patients. Am J Clin Pathol. 2003;119:374–380. doi: 10.1309/1kf54l6rb625556w. [DOI] [PubMed] [Google Scholar]
- 26.Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002;24:151–156. doi: 10.1002/hed.10040. [DOI] [PubMed] [Google Scholar]
- 27.Wikström P, Lissbrant IF, Stattin P, Egevad L, Bergh A. Endoglin (CD105) is expressed on immature blood vessels and is a marker for survival in prostate cancer. Prostate. 2002;51:268–275. doi: 10.1002/pros.10083. [DOI] [PubMed] [Google Scholar]
- 28.Yagasaki H, Kawata N, Takimoto Y, Nemoto N. Histopathological analysis of angiogenic factors in renal cell carcinoma. Int J Urol. 2003;10:220–227. doi: 10.1046/j.0919-8172.2003.00608.x. [DOI] [PubMed] [Google Scholar]
- 29.Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995;13:765–782. doi: 10.1200/JCO.1995.13.3.765. [DOI] [PubMed] [Google Scholar]
- 30.Cui S, Hano H, Sakata A, Harada T, Liu T, Takai S, Ushigome S. Enhanced CD34 expression of sinusoid-like vascular endothelial cells in hepatocellular carcinoma. Pathol Int. 1996;46:751–756. doi: 10.1111/j.1440-1827.1996.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 31.Anthony PP, Ramani P. Endothelial markers in malignant vascular tumours of the liver: superiority of QB-END/10 over von Willebrand factor and Ulex europaeus agglutinin 1. J Clin Pathol. 1991;44:29–32. doi: 10.1136/jcp.44.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasparini G. Clinical significance of determination of surrogate markers of angiogenesis in breast cancer. Crit Rev Oncol Hematol. 2001;37:97–114. doi: 10.1016/s1040-8428(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 33.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the 'angiogenesis progression' hypothesis. Eur J Cancer. 1996;32A:2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 34.Weidner N. Tumour vascularity and proliferation: clear evidence of a close relationship. J Pathol. 1999;189:297–299. doi: 10.1002/(SICI)1096-9896(199911)189:3<297::AID-PATH434>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg F, Röhrborn HJ, Otto T, Scheufler KM, Streffer C. NIR reflection measurements of hemoglobin and cytochrome aa3 in healthy tissue and tumors. Correlations to oxygen consumption: preclinical and clinical data. Adv Exp Med Biol. 1997;428:69–77. doi: 10.1007/978-1-4615-5399-1_11. [DOI] [PubMed] [Google Scholar]
- 36.Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977;13:399–408. doi: 10.1016/0026-2862(77)90106-6. [DOI] [PubMed] [Google Scholar]
- 37.Tannock IF, Steel GG. Quantitative techniques for study of the anatomy and function of small blood vessels in tumors. J Natl Cancer Inst. 1969;42:771–782. [PubMed] [Google Scholar]


