Fig. 4. A Conserved Signaling Pathway for Cell Polarity is Used by β2 Integrin to Induce Granule Polarization.
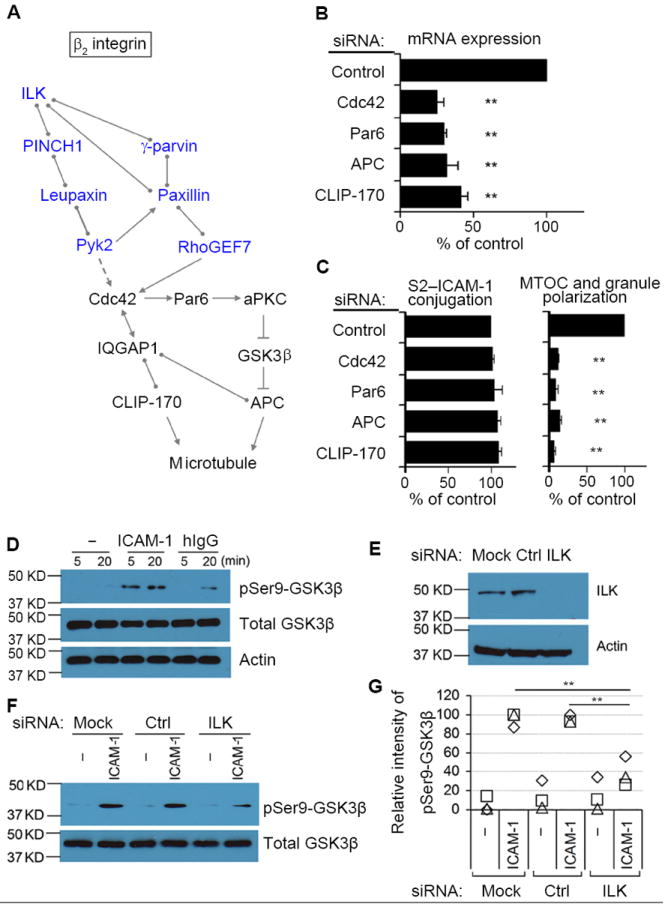
(A) Potential pathway for β2 integrin-dependent granule polarization in NK cells. Molecules in blue have been identified by mass spectrometry. Cdc42, known as a master regulator of microtubule-dependent cell polarity, can be activated by RhoGEF7 and Pyk2. Cdc42 controls cell polarity through both CLIP-170 and APC. Interactions in the diagram indicate direct binding (circles), activation (arrows), indirect activation (dashed arrow), or inhibition (cross bar), according to Ingenuity Pathway Analysis. (B) mRNA abundance after silencing of the indicated proteins. (C) Conjugation with S2–ICAM-1 cells and MTOC and granule polarization after silencing of the indicated proteins. Conjugation varied from 30% to 36%, and MTOC and granule polarization varied from 33% to 37% in the controls. Graphs show mean ± SEM from 3 experiments. **p<0.01. (D) Primary NK cells stimulated with BSA only (–), ICAM-1, or human IgG1 for the indicated times. Cell lysates were immunoblotted with antibodies to Ser9 phosphorylated GSK3β, total GSK3β, and actin. (E) ILK abundance monitored by immunoblot after silencing with siRNA. (F) After stimulation with BSA only (–) or ICAM-1 for 5 min, lysates were immunoblotted as in (D). (G) Intensity of GSK3β Ser9 phosphorylation, relative to phosphorylation in siRNA controls. Squares, triangles, and diamonds represent data from, from 3 independent experiments. **p<0.01.
