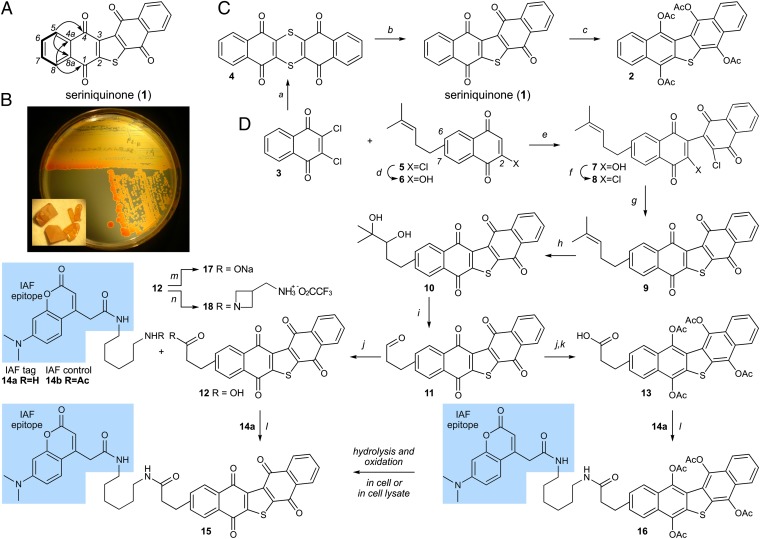
Fig. 1.
Isolation and chemical synthesis. (A) Structure of dinaphtho[2,3-b:2',3′-d]thiophene-5,7,12,13-tetraone or seriniquinone (1). Summary of salient NMR data including 1H-1H COSY (bold lines) and heteronuclear multiple-bond correlation spectroscopy correlations (arrows) observed in solutions of 1 in CDCl3. (B) Images depicting the marine bacterium Serinicoccus sp. strain CNJ927 growing on a nutrient agar surface, and crystals of 1 (Inset). Culture broths of Serinicoccus sp., strain CNJ927, provided 0.067 mg/L of 1. (C) Repetition of the published synthesis of 1 (13) confirmed the structure of the natural product. Tetra-acetate hydroquinone 2 was prepared as a prodrug motif, which when absorbed in cells underwent acetate hydrolysis followed by oxidation to 1 (SI Appendix, Fig. S2). (D) Advance of synthetic methods permitted access to IAF probes 15 and 16, as well as derivatives 10, 12, 17, and 18. Reagents and conditions: (a) dithiooxamide, Et3N, N,N-dimethylformamide (DMF), 50 °C, 10 h, 85%; (b) 30% aq. H2O2, AcOH, reflux, 3 h, 86%; (c) Zn dust, Ac2O, reflux, 2 h, 67%; (d) NaOH, MeOH, 2 h; (e) CsCO3, CH3CN, rt, 72 h; (f) (ClCO)2, DMF, CH2Cl2, 2 h, 49% (over 3 steps from 5); (g) Na2S, aq. THF, rt, 1h; (h) K2OsO4 • 2 H2O, N-methylmorpholine oxide, THF, H2O, 16 h, 67% (over 2 steps from 8); (i) NaIO4 on SiO2, CH2Cl2, 2 h, 98%; (j) oxone, DMF, 2 h, 98%; (k) Zn dust, Ac2O, reflux, 2 h, 65%; (l) 1-[bis(dimethylamino) methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), EtNiPr2, DMF, rt, 2 h, 29% for 16; (m) NaOMe, MeOH, 16 h, 24%; (n) tert-butyl acetidine-3-ylmethylcarbamate, HATU, EtNiPr2, DMF, rt, 2 h, then TFA, CH2Cl2, 1 h, 42% (two steps). Starting material 5 was obtained as batch-dependent inseparable 5:2–6:1 mixture of 2-chloro-7-(4-methylpent-3-en-1-yl)naphthalene-1,4-dione (major) and 2-chloro-6-(4-methylpent-3-en-1-yl)naphthalene-1,4-dione (minor).