Abstract
Objective: We conducted meta-analyses of functional and structural neuroimaging studies comparing adolescent and adult individuals with a history of suicidal behavior and a psychiatric disorder to psychiatric controls in order to objectify changes in brain structure and function in association with a vulnerability to suicidal behavior.
Methods: Magnetic resonance imaging studies published up to July 2013 investigating structural or functional brain correlates of suicidal behavior were identified through computerized and manual literature searches. Activation foci from 12 studies encompassing 475 individuals, i.e., 213 suicide attempters and 262 psychiatric controls were subjected to meta-analytical study using anatomic or activation likelihood estimation (ALE).
Result: Activation likelihood estimation revealed structural deficits and functional changes in association with a history of suicidal behavior. Structural findings included reduced volumes of the rectal gyrus, superior temporal gyrus and caudate nucleus. Functional differences between study groups included an increased reactivity of the anterior and posterior cingulate cortices.
Discussion: A history of suicidal behavior appears to be associated with (probably interrelated) structural deficits and functional overactivation in brain areas, which contribute to a decision-making network. The findings suggest that a vulnerability to suicidal behavior can be defined in terms of a reduced motivational control over the intentional behavioral reaction to salient negative stimuli.
Keywords: suicide, vulnerability, meta-analysis, gray matter, decision-making
INTRODUCTION
It is estimated that one million people commit suicide annually (World Health Organisation [WHO], 2002). Non-fatal suicidal behavior occurs 10–20 times more frequently, and a history of self-harm or suicide attempts is the strongest risk factor for suicide, being present in at least 40% of cases (Hawton and van Heeringen, 2009). Human and societal costs of suicidal behavior are substantial, and many countries have recently developed suicide prevention programs. The prevention of suicide, however, still poses major challenges. Clinicians are unable to predict the occurrence of suicidal behavior. In addition, when suicide risk is considered high, its management proves challenging because of the poor evidence base. It is, for example, impossible to predict whether an individual at risk will respond to treatment with a decrease or an increase in suicide risk. Even if there is a positive response to treatment, it is often unclear how and why this happened. Suicide prevention is thus in great need of markers that predict suicidal behavior and serve as a substrate for treatment.
Based upon the current state of knowledge, a stress-diathesis or stress-vulnerability model of suicidal behavior has been developed (Van Heeringen, 2012). In general, stress-vulnerability models describe the mechanisms due to which diathetic or vulnerable individuals respond with abnormal or pathological reactions to physiological stimuli or the ordinary conditions of life that are borne by the majority of individuals without injury (Zuckerman, 1999). With regard to suicidal behavior, stressors including interpersonal, professional, or financial problems commonly precipitate these behaviors. In addition, suicidal behavior occurs, in general, in the context of psychiatric conditions such as depression or bipolar disorder. However, only a small proportion of individuals confronted with stressors and suffering from these disorders will actually show suicidal behavior. The stress-vulnerability model of suicidal behavior may thus explain such behavior as the consequence of an interaction between exposure to stressors and a vulnerability in individuals suffering from psychiatric disorders. Comparing individuals with a history of a psychiatric disorder and suicidal behavior to those with a history of such a psychiatric disorder but no history of suicidal behavior can thus be expected to shed light on the particular vulnerability to suicidal behavior.
Evidence of a neurobiological basis of the vulnerability to suicidal behavior is increasing (van Heeringen and Mann, 2014). From a neurochemical point of view, the serotonin neurotransmission system and the HPA-stress-response system appear to be crucially involved. At a neuroanatomical level, postmortem and neuroimaging studies show changes in a number of areas in the brain in association with a vulnerability to suicidal behavior. Four reviews of neuroimaging studies have recently been published (Desmyter et al., 2011; Jollant et al., 2011; van Heeringen et al., 2011; Zhang et al., 2014). These reviews agreed on the involvement of particular brain areas in the development of suicidal behavior, including the dorsolateral and orbitofrontal cortex. However, no clear picture emerged from the reviews with regard to other cortical regions and subcortical involvement. Moreover, sample sizes of studied patient groups were small, which hampers the interpretation of findings.
In order to investigate whether the subjective conclusions from the reviews can be objectified, and, more importantly, to specifically study the vulnerability to suicidal behavior using neuroimaging, we performed coordinate-based meta-analyses of structural and functional neuroimaging studies (CBMA; Turkeltaub et al., 2002). The objective of CBMA is not to estimate the magnitude of an effect across studies, but rather to identify anatomical locations in which an effect is observed consistently. By including only studies in which brain structure or function of individuals with a history of a psychiatric disorder and suicidal behavior were compared to brain structure or function of those with a history of the psychiatric disorder but no suicidal behavior, this meta-analysis aimed at investigating particularly the association between a vulnerability to suicidal behavior and brain structure or function.
MATERIALS AND METHODS
DATA SEARCH
A search was carried out using Web of Science, PsycINFO, and Pubmed databases with the keywords: suicide, suicidal, neuroimaging, magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission tomography (SPET), single photon emission computerized tomography (SPECT), voxel based morphometry (VBM). Criteria for including studies in the meta-analyses were:
(1) Peer-reviewed original structural or functional brain imaging study published up to July 2013.
(2) Use of PET, SPE(C)T or MRI.
(3) Contrast between individuals with a history of a psychiatric disorder and suicidal behavior and individuals with a history of the psychiatric disorder but no suicidal behavior
(4) Adolescents and adults.
(5) Results reported as coordinates in a normalized standard stereotactic space, i.e., the Talairach or Montreal Neurological Institute (MNI) reference system.
Unpublished studies, case reports or conference abstracts were not included, as were studies reporting only region-of-interest findings or using seed-voxel-based analysis procedures. Studies comparing elderly suicide attempters to elderly psychiatric controls were not included in the meta-analysis, because the neurobiology of suicidal behavior may differ between the elderly and adolescents or adults. The reference lists of relevant papers were checked manually for additional relevant publications not previously identified.
The literature search identified articles reporting on 22 structural and 16 functional brain imaging studies, in which individuals with a history of suicide attempts were included and compared to controls.
Seven structural imaging studies were excluded because Talairach or MNI coordinates of identified anatomical structures were not reported (Monkul et al., 2007; Matsuo et al., 2010; Vang et al., 2010; Cyprien et al., 2011; Spoletini et al., 2011; Nery-Fernandes et al., 2012; Giakoumatos et al., 2013). Two studies were excluded because the study population consisted of elderly (Hwang et al., 2010; Dombrovski et al., 2012). Two additional studies were excluded because results for suicide attempters were not reported separately as attempters were regarded as part of a larger group of individuals considered at increased risk of suicide (Wagner et al., 2011, 2012). Finally, five studies were excluded from the meta-analysis because they focused specifically on hyperintensities, i.e., white matter hyperintensities (Ahearn et al., 2001) or gray matter hyperintensities (Ehrlich et al., 2004, 2005; Pompili et al., 2007, 2008).
Identified functional imaging studies used SPECT (n = 4; Audenaert et al., 2001, 2002; Lindstrom et al., 2004; Ryding et al., 2006), PET (n = 4; Meyer et al., 2003; Oquendo et al., 2003; Leyton et al., 2006; Nye et al., 2013), and fMRI (n = 8; Jollant et al., 2008, 2010; Pan et al., 2011, 2013a,b; Marchand et al., 2012; Dombrovski et al., 2013; Fan et al., 2013). A number of studies had to be excluded from the meta-analysis due to absence of psychiatric controls (Audenaert et al., 2001, 2002; Oquendo et al., 2003; Leyton et al., 2006), or the absence of stereotactic coordinates in the report (Meyer et al., 2003; Lindstrom et al., 2004; Ryding et al., 2006; Nye et al., 2013). One study was not included because the analyses focused only on striatal and cortical midline structures (Marchand et al., 2012), while another study was excluded as the study population consisted of elderly individuals (Dombrovski et al., 2013).
ALE META-ANALYSIS
Reported coordinates were analyzed for topographic convergence using the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Turkeltaub et al., 2002; Eickhoff et al., 2009). The goal of coordinate-based meta-analyses of neuroimaging data is to identify brain areas where the reported foci of activation converge across published experiments. To this end, the meta-analysis determines if the clustering is significantly higher than expected under the null distribution of a random spatial association of results from the considered experiments while acknowledging the spatial uncertainty associated with neuroimaging foci. As the first step, and following conversion from MNI space to Talairach space using icbm2tal where needed, reported foci were interpreted as centers for 3D Gaussian probability distributions that capture the spatial uncertainty associated with each focus. This uncertainty is mostly a function of between-template (attributable to different normalization strategies and templates across laboratories) and between-subject (due to small sample sizes) variance. In fact, the between-template and between-subject variability are acknowledged based on empirical estimates, the latter being additionally gaged by individual sample size (Eickhoff et al., 2009). In a second step, the probabilities of all activation foci in a certain experiment were combined for each voxel, yielding a modeled activation (MA) map (Turkeltaub et al., 2012). Voxel-wise ALE scores resulted from the union across these MA maps and quantified the convergence across experiments at each particular location in the brain. The third and last step distinguished between random and “true” convergence by comparing the ensuing ALE scores against an empirical null distribution reflecting a random spatial association between the experiments’ MA maps (Eickhoff et al., 2012). The within-experiment distribution of foci, however, was regarded as fixed (Eickhoff et al., 2009). Thus, a random-effects inference was invoked, focusing on the above-chance convergence across different experiments (Eickhoff et al., 2009; Caspers et al., 2010; Kurth et al., 2010). The resulting ALE scores were tested against the earlier calculated “true” ALE scores and cut off at a cluster-level-corrected threshold of p < 0.05. The cluster size threshold was >200 mm3.
RESULTS
STRUCTURAL IMAGING STUDIES
As shown in Table 1, six structural imaging studies were included in the meta-analysis (Aguilar et al., 2008; Rusch et al., 2008; Jia et al., 2010; Benedetti et al., 2011; Mahon et al., 2012; Soloff et al., 2012).
Table 1.
Structural imaging studies meeting inclusion criteria.
| Study (first author) | Year | Imaging modality | Study population SA/PC/HC | Mean age years | % F | Measurement | Available data |
|---|---|---|---|---|---|---|---|
| Aguilar | 2008 | MRI 1,5T | S 13/24/0 | 37/43/0 | 0/0/0 | VBM | BA; x, y, z; T; p |
| Rüsch | 2008 | MRI 1,5T | S 10/45/55 | 30/37/36 | 30/40/38 | VBM | x, y, z; p; absolute volume (IU); F; p |
| Jia | 2010 | MRI 3T | MD 16/36/52 | 34/35/37 | 69/44/54 | DTI FA | x, y, z; cluster size; F; t |
| Benedetti | 2011 | MRI 3T | BD 19/38/0 | 44/46/0 | 56/72/0 | VBM | BA; x, y, z; cluster size; Z; p |
| Mahon | 2012 | MRI 1,5T | BD 14/15/15 | 33/37/34 | 36/40/47 | DTI FA | x, y, z; cluster size; p |
| Soloff | 2012 | MRI 1,5T | BPD 44/24/0 | 30/26/26 | 82/67/46 | VBM | x, y, z; cluster size; p |
SA, suicide attempters; PC, psychiatric controls; HC, healthy controls; S, schizophrenia; MD, major depression; BD, bipolar disorder; BPD, borderline personality disorder; VBM, voxel-based morphometry; DTI, diffusion tensor imaging; FA, fractional anisotropy; SB, suicidal behaviour; BA, Brodmann area; x, y, z, stereotactic coordinates.
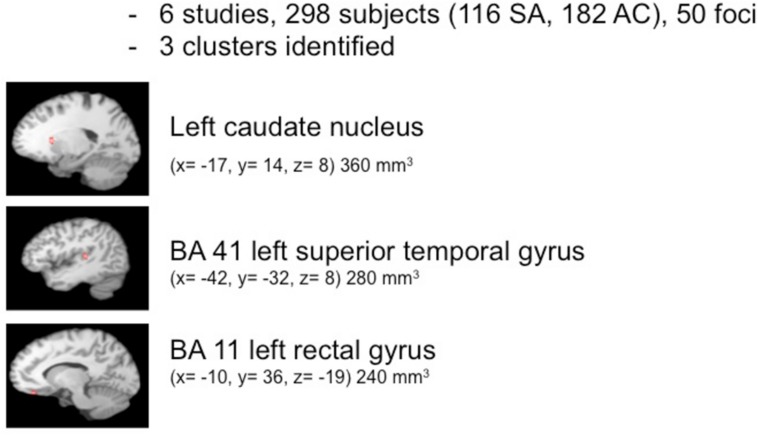
The pooled study population of the six structural imaging studies in the meta-analysis consisted of 298 individuals, i.e., 116 suicide attempters, and 182 psychiatric controls. The total number of foci identified in the studies was 50. As shown in Figure 1, ALE analysis identified three clusters indicating reduced gray matter volume in the left hemisphere, i.e., in the caudate nucleus (cluster size: 360 mm3; center: x = -16,75, y = 13,77, z = 8,46), the superior temporal gyrus (cluster size: 280 mm3; center: x = -41,49, y = -31,9, z = 8,14), and the rectal gyrus (cluster size: 240 mm3; center: x = -9,93, y= 35,81, z = -19,08).
FIGURE 1.
Meta-analysis of structural imaging studies.
FUNCTIONAL IMAGING STUDIES
As shown in Table 2, six studies were included in the meta-analysis (Jollant et al., 2008, 2010; Pan et al., 2011, 2013a,b; Fan et al., 2013).
Table 2.
Functional imaging studies included in the meta-analysis.
| Study (first author) | Year | Imaging modality | Study population SA/PC/HC | Mean age years | % F | Measurement | Available data |
|---|---|---|---|---|---|---|---|
| Jollant | 2008 | fMRI 1,5T | MD 13/14/16 | 40/44/32 | 0/0/0 | facial emotion recognition | BA; x, y, z; volume (voxels); p |
| Jollant | 2010 | fMRI 1,5T | MD 13/12/- | 38/43/30 | 0/0/0 | IGT | BA; x, y, z; U; p |
| Pan | 2011 | fMRI 3T | MD 15/15/14 | 16/16/15 | 73/53/43 | go/no-go | BA; x, y, z; F; Z; k (cluster); p |
| Pan | 2013a | fMRI 3T | MD 15/14/13 | 16/16/15 | 73/50/38 | IGT | BA; x, y, z; F; Z; k (cluster); p |
| Fan | 2013 | fMRI 3T | MD 27/10/57 | 34/38/37 | 59/56/55 | ALFF | BA; x, y, z; K (cluster number); t |
| Pan | 2013b | fMRI 3T | MD 14/15/15 | 16/16/15 | 71/53/47 | angry & happy faces | BA; x, y, z; F; Z; k (cluster); t; p |
SA, suicide attempters; PC, psychiatric controls; HC, healthy controls; MD, major depression; IGT, iowa gambling task; ALFF, amplitude of low-frequency fluctuation; BA, Brodmann area; x, y, z, stereotactic coordinates.
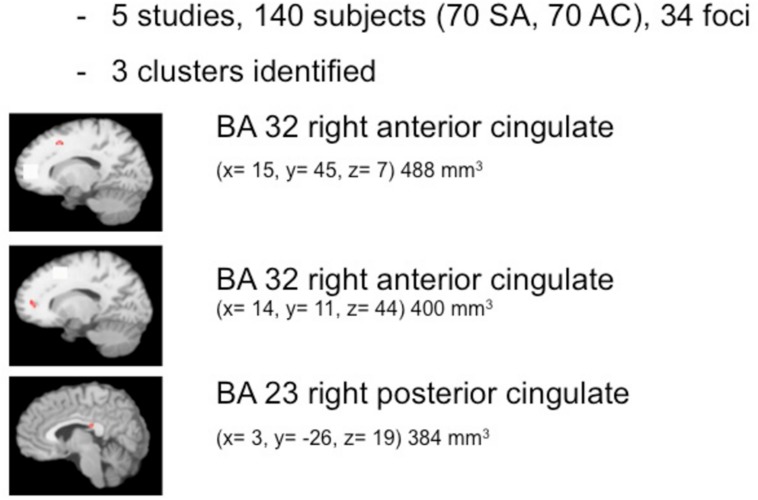
The pooled study population of the included functional imaging studies in the meta-analysis consisted of 177 individuals, i.e., 97 suicide attempters, and 80 psychiatric controls. The number of foci identified in the studies was 34. As shown in Figure 2, ALE analysis identified three clusters in the right hemisphere, i.e., two clusters in the right anterior cingulate and one in the posterior cingulate. Characteristics of the clusters in the right anterior cingulate were (1) cluster size: 480 mm3; center: x= 14,42, y = 44,92, z = 7,28 (dorsal anterior cingulate), and (2) cluster size: 400 mm3; center: x= 13,73, y = 11,28, z = 43,55 (rostral anterior cingulate). The size of the cluster in the right posterior cingulate was 384 mm3, centered at x = 2,97, y = -25,94, z = 19,39. The cluster in the dorsal anterior cingulate showed increased activation in suicide attempters when compared to psychiatric controls during exposure to angry faces or mild happy faces, while activation was relatively less increased in attempters than in psychiatric controls during high-risk decisions. The cluster in the rostral anterior cingulate showed increased activation in suicide attempters when compared to psychiatric controls during exposure to angry faces, while activation was relatively less increased in attempters than in psychiatric controls during a go/no-go task. Finally, the cluster in the posterior cingulate showed increased activation in suicide attempters when compared to psychiatric controls during exposure to happy faces, while activation was relatively less increased in attempters than in psychiatric controls during high-risk decisions.
FIGURE 2.
Meta-analysis of functional imaging studies.
DISCUSSION
This is the first meta-analysis of imaging studies of suicidal behavior. We were particularly interested in studying brain correlates of a vulnerability for suicidal behavior, and therefore included only studies, which reported a comparison between individuals with a history of a psychiatric disorder and a history of suicide attempts to individuals with a history of only this psychiatric disorder.
The findings support the existence of a vulnerability to suicidal behavior and suggest a neuroanatomical basis for this diathesis. In summary, ALE meta-analyses of 12 studies identifies six regions in the brain that in association with a history of suicidal behavior are characterized by decreased volumes or changes in reactivity to emotional and cognitive stimuli. The structural correlate of the vulnerability consisted of clusters of smaller volumes in the left superior temporal gyrus, rectal gyrus, and caudate nucleus. Functional correlates of the vulnerability to suicidal behavior were confined to the right cingulate gyrus, with two clusters in the anterior cingulate and one in the posterior cingulate.
Preceding a discussion of these findings a number of methodological issues need to be addressed. The number of studies, which could be included in the meta-analyses, is rather small. Due to methodological and ethical difficulties, inclusion of suitable suicidal patients in neurobiological research is a difficult process, so that it will take a substantial amount of time before a larger number of studies will be available for meta-analyses. The inclusion of a limited number of functional imaging studies implies that the effects of only a small number of activation paradigms have been investigated in suicide attempters. Therefore, it cannot be concluded that a diathesis to suicidal behavior is characterized by changed cingulate reactivity to particular facial emotional expressions or cognitive stimuli. Changes in such reactivity may also occur following exposure to other emotional or cognitive stimuli. In addition, it should be noted that studies were variable in terms of applied definitions of suicidal behavior and psychiatric diagnoses in suicide attempters and controls. The neurobiology of suicidal behavior along with depression may differ from that along with schizophrenia, but evidence of a shared underlying vulnerability is increasing (van Heeringen and Mann, 2014). As imaging studies involving elderly suicide attempters and controls were not included in the meta-analysis, the results of the current meta-analyses may not be applicable to the elderly. The conclusion that the demonstrated changes in brain functions and structures reflect a vulnerability to suicidal behavior is based on the assumption that suicide attempters and controls did not differ with regard to state-dependent characteristics. An effect of such state-dependent characteristics, including the use of medication, is most probably limited due to the strict inclusion criteria, but cannot be ruled out completely. Even with the strict inclusion criteria, the suitable studies still varied on a large number of variables such as statistical thresholds, smoothing kernels, registration procedures to standard space, and MRI scanning parameters. While current coordinate-based meta-analysis methods cannot account for all these differences separately, ALE does estimate a spatial uncertainty per individual study thereby alleviating some of the between-studies variability arising from varying study specific parameters such as the number of subjects or the use of different brain templates (Eickhoff et al., 2009). Finally, disturbances in brain functions may be attributable to structural changes. The differences between structurally and functionally affected regions in the current study, however, suggest that functional changes in association with a diathesis to suicidal behavior are not due to structural disturbances, at least not in the same areas. The relationship between the structural and functional findings will be discussed below.
With regard to the structural findings, combining voxel-based DTI and gray matter data in one meta-analysis may answer fundamental questions such as whether gray and white matter abnormalities, when found, are consistent with one another, and whether white matter alterations are consistent with alterations in gray matter of areas connected by these white matter abnormalities. The current meta-analysis, however, did not identify abnormalities in white matter in association with a history of suicidal behavior. A decreased gray matter volume of the orbitofrontal cortex has been shown in several psychiatric disorders, including depression (Wagner et al., 2008) and anxiety disorders (Strawn et al., 2013). Reduced volumes of the superior temporal gyrus have been demonstrated in schizophrenia (Palaniyappan et al., 2012). Reduced caudate volumes have been found in, among others, bulimia nervosa (Amianto et al., 2013), borderline personality disorder (O’Neill et al., 2013) and depression (Ma et al., 2012). These disorders also share a substantially increased suicide risk (Hawton and van Heeringen, 2009).
At first glance, the structural findings from the meta-analysis reflect a complex pattern of changes, and each of the involved regions may serve multiple functions. However, recent research findings point at a converging function from a cognitive neuroscience point of view, i.e., the processing of negative emotions. The superior temporal gyrus (Radua et al., 2010; Kumfor et al., 2013), the rectal gyrus (Schoenbaum et al., 2011; Szatkowska et al., 2011) and the caudate nucleus (Kemp et al., 2013) are involved in emotion processing, particularly with regard to negative emotions as shown in studies of facial emotion perception. The structures identified as structural correlates of the vulnerability to suicidal behavior appear to be particularly involved in the processing of the punishing aspect of salient events and may thus mediate in planning behavior on the basis of negative information.
The putative role of disturbed emotion processing, resulting in the aberrant salience of particular emotional stimuli, in the vulnerability to suicidal behavior is confirmed by the findings from the meta-analysis of functional imaging studies of suicidal behavior. Two clusters with changed activation patterns in association with a history of suicidal behavior were identified in the ACC, which is a structure in the medial prefrontal cortex that comprises several functional subdivisions. Rostral regions of the ACC (rACC) activate during emotional states or during tasks that involve interference from emotional stimuli. In contrast, dorsal regions of the ACC (dACC) activate during tasks that involve interference from non-emotional stimuli. The current meta-analysis shows increased activation during emotional tasks (i.e., exposure to emotional faces) and decreased activation during cognitive tasks [i.e., the iowa gambling task (IGT) and a Go/No-go task, respectively] in the rostral and in the dorsal ACC in association with a history of suicidal behavior. A distinction between rostral/emotional and dorsal/non-emotional is thus not found in this study, which is in keeping with recent models of functional organization of the brain (Lindquist and Barrett, 2012). A third cluster was identified in the PCC, in a similar way showing increased activation upon perception of emotional faces and decreased activation during the IGT.
The mechanism, by means of which reduced volumes of particular brain areas relate to changes in functional cognitive emotional characteristics, is yet unclear. Structural abnormalities may represent a trait factor and lead to functional changes that represent state factors (de Kwaasteniet et al., 2013). However, only very few studies have focused on the association between structural and functional cognitive or emotional alterations in the brain in the context of psychiatric disorders. No such studies exist with regard to suicidal behavior. Wagner et al. (2008) demonstrated a significant negative correlation between gray matter volume in the gyrus rectus and the BOLD signal in the rACC during the Stroop task in depressed individuals. Scheuerecker et al. (2010) reported a similar negative correlation between Brodmann area 11 (i.e., rectal gyrus) volumes and changes in the BOLD signal in, among others, the left caudate nucleus during an emotional face-matching task. The findings suggest that due to decreased gray matter in the orbitofrontal cortex depressed patients are not able to suppress interfering rACC and caudate activity. As the rACC and caudate are considered part of the brain’s default mode network (DMN), the authors suggest that the inability to deactivate this network during cognitive processing is related to structural deficits in the rectal gyrus. Thus, a dysbalance of the orbitofrontal-cingulate network in controlling maladaptive affective responses during cognitive processing is strongly related to structural lesions within this network (Wagner et al., 2008). As the current meta-analysis showed similar functional disturbances in another part of the DMN, i.e., the PCC, the findings suggest that a vulnerability to suicidal behavior is associated with disturbances in an orbitofrontal- cingulate network, characterized by the interrelated inability to control maladaptive responses during cognitive processing and a reduced volume of the rectal gyrus. Grimm et al. (2009) provided further support for a role of reduced task-induced rACC DMN deactivation in suicidal behavior in depressed individuals by reporting a correlation between decreased negative BOLD responses in the DMN during emotion processing and feelings of hopelessness, a major risk factor for suicide. Thus, the current findings suggest that the increased salience of particular negative stimuli and the inability to control maladaptive responses during cognitive processing due to structural deficits are two core characteristics of the vulnerability to suicidal behavior.
Further insight in the nature of the deficits in cognitive processing associated with a vulnerability to suicidal behavior is provided by findings from neuropsychological studies. A recent meta-analysis showed a particular role of deficits in decision-making, verbal fluency and Stroop interference in this respect (Richard-Devantoy et al., 2014). While the currently demonstrated involvement of the ACC in a vulnerability to suicidal behavior may explain the lower Stroop performance, the findings from the current meta-analyses may particularly shed light on the role of deficient decision-making. The caudate, OFC and ACC are implicated in the process of reinforcement-guided decision-making, but appear to make distinctive contributions. In conjunction with the caudate, OFC codes the stimulus that is target of the action in terms of specific reward expectations, thus determining the tendency to action, i.e., to approach or avoid the predictive stimulus. Delay is an important aspect of relevant information for determining reward expectation. While coding of short-term reward expectation occurs in conjunction with the ventral striatum-based reward system, the dorsal striatum including the caudate nucleus appears to be involved in predicting future reward (Tanaka et al., 2004, 2007; Onoda et al., 2011). The dorsal striatum and its connected cortical control network thus enact motivational control over intentional behavior (Harsay et al., 2011). In keeping with this line of reasoning, a reduced caudate volume was recently found associated with increased delay discounting in Parkinson patients (Szamosi et al., 2013). Given the well-documented involvement of serotonin disturbances in suicidal behavior (Mann, 2013), it is of importance to note that striatal reward prediction at different time scales is modulated by the central serotonergic system (Tanaka et al., 2007).
Taken together, the findings from meta-analyses of neuroimaging and neuropsychological studies thus suggest that the vulnerability to suicidal behavior can be defined in terms of a reduced motivational control over the intentional behavioral reaction to salient negative stimuli. The current meta-analyses have identified structural abnormalities that may represent the vulnerability trait factor, leading to functional changes that may represent the state factors. Further study is needed to confirm these findings and explore network and connectivity characteristics of identified changes in neural substrates, the causes of these changes, which may be genetic or acquired, and the effects of treatments such as antidepressants, rTMS, ketamine, and psychotherapy on structural and functional changes.
ACKNOWLEDGMENT
The authors have neither financial interest in, nor financial support for writing this meta-analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Aguilar E. J., Garcia-Marti G., Marti-Bonmati L., Lull J. J., Moratal D., Escarti M. J., et al. (2008). Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 32 1673–1676. 10.1016/j.pnpbp.2008.06.016 [DOI] [PubMed] [Google Scholar]
- Ahearn E. P., Jamison K. R., Steffens D. C., Cassidy F., Provenzale J. M., Lehman A., et al. (2001). MRI correlates of suicide attempt history in unipolar depression. Biol. Psychiatry 50 266–270. 10.1016/S0006-3223(01)01098-8 [DOI] [PubMed] [Google Scholar]
- Amianto F., Caroppo P., D’Agata F., Spalatro A., Lavagnino L., Caglio M., et al. (2013). Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a Voxel-based morphometry study. Psychiatry Res. 213 210–216. 10.1016/j.pscychresns.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Audenaert K., Goethals I., Van Laere K., Lahorte P., Brans B., Versijpt J., et al. (2002). SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nucl. Med. Commun. 23 907–916. 10.1097/00006231-200209000-00015 [DOI] [PubMed] [Google Scholar]
- Audenaert K., Van Laere K., Dumont F., Slegers G., Mertens J., van Heeringen C., et al. (2001). Decreased frontal serotonin 5-HT2a receptor binding index in deliberate self-harm patients. Eur. J. Nucl. Med. 28 175182. 10.1007/s002590000392 [DOI] [PubMed] [Google Scholar]
- Benedetti F., Radaelli D., Poletti S., Locatelli C., Falini A., Colombo C., et al. (2011). Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J. Affect. Disord. 135 139–147. 10.1016/j.jad.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A. R., Eickhoff S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50 1148–1167. 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyprien F., Courtet P., Malafosse A., Maller J., Meslin C., Bonafe A., et al. (2011). Suicidal behavior is associated with reduced corpus callosum area. Biol. Psychiatry 70 320–326. 10.1016/j.biopsych.2011.02.035 [DOI] [PubMed] [Google Scholar]
- de Kwaasteniet B., Ruhe E., Caan M., Rive M., Olabarriaga S., Groefsema M., et al. (2013). Relation between structural and functional connectivity in major depressive disorder. Biol. Psychiatry 74 40–47. 10.1016/j.biopsych.2012.12.024 [DOI] [PubMed] [Google Scholar]
- Desmyter S., van Heeringen C., Audenaert K. (2011). Structural and functional neuroimaging studies of the suicidal brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 35 796–808. 10.1016/j.pnpbp.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Dombrovski A. Y., Siegle G. J., Szanto K., Clark L., Reynolds C. F., Aizenstein H. (2012). The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol. Med. 42 1203–1215. 10.1017/S0033291711002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski A. Y., Szanto K., Clark L., Reynolds C. F., Siegle G. J. (2013). Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry 70 1020–1030. 10.1001/jamapsychiatry.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S., Breeze J. L., Hesdorffer D. C., Noam G. G., Hong X. N., Alban R. L., et al. (2005). White matter hyperintensities and their association with suicidality in depressed young adults. J. Affect. Disord. 86 281–287. 10.1016/j.jad.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Ehrlich S., Noam G. G., Lyoo I. K., Kwon B. J., Clark M. A., Renshaw P. F. (2004). White matter hyperintensities and their associations with suicidality in psychiatrically hospitalized children and adolescents. J. Am. Acad. Child Psychiatry 43 770–776. 10.1097/01.chi.0000120020.48166.93 [DOI] [PubMed] [Google Scholar]
- Eickhoff S. B., Bzdok D., Laird A. R., Kurth F., Fox P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59 2349–2361. 10.1016/j.neuroimage.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S. B., Laird A. R., Grefkes C., Wang L. E., Zilles K., Fox P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30 2907–2926. 10.1002/Hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. T., Wu X., Yao L., Dong J. (2013). Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci. Lett. 534 35–40. 10.1016/j.neulet.2012.11.032 [DOI] [PubMed] [Google Scholar]
- Giakoumatos C. I., Tandon N., Shah J., Mathew I. T., Brady R. O., Clementz B. A., et al. (2013). Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J. Psychiatr. Res. 47 1389–1395. 10.1016/j.jpsychires.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Boesiger P., Beck J., Schuepbach D., Bermpohl F., Walter M., et al. (2009). Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacol. 34 932–943. 10.1038/Npp.2008.81 [DOI] [PubMed] [Google Scholar]
- Harsay H. A., Cohen M. X., Oosterhof N. N., Forstmann B. U., Mars R. B., Ridderinkhof K. R. (2011). Functional connectivity of the striatum links motivation to action control in humans. J. Neurosci. 31 10701–10711. 10.1523/Jneurosci.5415-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K., van Heeringen K. (2009). Suicide. Lancet 373 1372–1381. 10.1016/S0140-6736(09)60372-X [DOI] [PubMed] [Google Scholar]
- Hwang J. P., Lee T. W., Tsai S. J., Chen T. J., Yang C. H., Lirng J. F., et al. (2010). Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J. Geriatr. Psychiatry Neurol. 23 171–184. 10.1177/0891988710363713 [DOI] [PubMed] [Google Scholar]
- Jia Z. Y., Huang X. Q., Wu Q. Z., Zhang T. J., Lui S., Zhang J. R., et al. (2010). High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am. J. Psychiatry 167 1381–1390. 10.1176/appi.ajp.2010.09101513 [DOI] [PubMed] [Google Scholar]
- Jollant F., Lawrence N. L., Olie E., Guillaume S., Courtet P. (2011). The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J. Biol. Psychia 12 319–339. 10.3109/15622975.2011.556200 [DOI] [PubMed] [Google Scholar]
- Jollant F., Lawrence N. S., Giampietro V., Brammer M. J., Fullana M. A., Drapier D., et al. (2008). Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am. J. Psychiatry 165 740–748. 10.1176/appi.ajp.2008.07081239 [DOI] [PubMed] [Google Scholar]
- Jollant F., Lawrence N. S., Olie E., O’Daly O., Malafosse A., Courtet P., et al. (2010). Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. Neuroimage 51 1275–1281. 10.1016/j.neuroimage.2010.03.027 [DOI] [PubMed] [Google Scholar]
- Kemp J., Berthel M. C., Dufour A., Despres O., Henry A., Namer I. J., et al. (2013). Caudate nucleus and social cognition: neuropsychological and SPECT evidence from a patient with focal caudate lesion. Cortex 49 559–571. 10.1016/j.cortex.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J. R., Piguet O. (2013). Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS ONE 8:e67457 10.1371/journal.pone.0067457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F., Zilles K., Fox P. T., Laird A. R., Eickhoff S. B. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M., Paquette V., Gravel P., Rosa-Neto P., Weston F., Diksic M., et al. (2006). Alpha-[C-11]Methyl-L-tryptophan trapping in the orbital and ventral medial prefrontal cortex of suicide attempters. Eur. Neuropsychopharmacol. 16 220–223. 10.1016/j.euroneuro.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Lindquist K. A., Barrett L. F. (2012). A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn. Sci. 16 533–540. 10.1016/j.tics.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom M. B., Ryding E., Bosson P., Ahnlide J. A., Rosen I., Traskman-Bendz L. (2004). Impulsivity related to brain serotonin transporter binding capacity in suicide attempters. Eur. Neuropsychopharmacol. 14 295–300. 10.1016/S0924-977x(03)00218-9 [DOI] [PubMed] [Google Scholar]
- Ma C. Q., Ding J. R., Li J., Guo W. B., Long Z. L., Liu F., et al. (2012). Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS ONE 7:e45263 10.1371/journal.pone.0045263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K., Burdick K. E., Wu J. H., Ardekani B. A., Szeszko P. R. (2012). Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord. 14 80–89. 10.1111/j.1399-5618.2012.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. J. (2013). The serotonergic system in mood disorders and suicidal behaviour. Philos. Trans. R Soc. B Lond. B Biol. Sci. 368 20120537 10.1098/Rstb.2012.0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand W. R., Lee J. N., Johnson S., Thatcher J., Gale P., Wood N., et al. (2012). Striatal and cortical midline circuits in major depression: implications for suicide and symptom expression. Prog. Neuropsychopharmacol. Biol. Psychiatry 36 290–299. 10.1016/j.pnpbp.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Matsuo K., Nielsen N., Nicoletti M. A., Hatch J. P., Monkul E. S., Watanabe Y., et al. (2010). Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci. Lett. 469 75–80. 10.1016/j.neulet.2009.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., McMain S., Kennedy S. H., Korman L., Brown G. M., DaSilva J. N., et al. (2003). Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am. J. Psychiatry 160 90–99. 10.1176/appi.ajp.160.1.90 [DOI] [PubMed] [Google Scholar]
- Monkul E. S., Hatch J. P., Nicoletti M. A., Spence S., Brambilla P., Lacerda A. L. T., et al. (2007). Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol. Psychiatry 12 360–366. 10.1038/sj.mp.4001919 [DOI] [PubMed] [Google Scholar]
- Nery-Fernandes F., Rocha M. V., Jackowski A., Ladeia G., Guimaraes J. L., Quarantini L. C., et al. (2012). Reduced posterior corpus callosum area in suicidal and non-suicidal patients with bipolar disorder. J. Affect. Disord. 142 150–155. 10.1016/j.jad.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Nye J. A., Purselle D., Plisson C., Voll R. J., Stehouwer J. S., Votaw J. R., et al. (2013). Decreased brainstem and putamen sert binding potential in depressed suicide attempters using [C-11]-zient pet imaging. Depress. Anxiety 30 902–907. 10.1002/Da.22049 [DOI] [PubMed] [Google Scholar]
- O’Neill A., D’Souza A., Carballedo A., Joseph S., Kerskens C., Frodl T. (2013). Magnetic resonance imaging in patients with borderline personality disorder: a study of volumetric abnormalities. Psychiatry Res. 213 1–10. 10.1016/j.pscychresns.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Onoda K., Okamoto Y., Kunisato Y., Aoyama S., Shishida K., Okada G., et al. (2011). Inter-individual discount factor differences in reward prediction are topographically associated with caudate activation. Exp. Brain Res. 212 593–601. 10.1007/s00221-011-2771-2773 [DOI] [PubMed] [Google Scholar]
- Oquendo M. A., Placidi G. P. A., Malone K. M., Campbell C., Keilp J., Brodsky B., et al. (2003). Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch. Gen. Psychiatry 60 14–22. 10.1001/archpsyc.60.1.14 [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Balain V., Radua J., Liddle P. F. (2012). Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr. Res. 137 169173. 10.1016/j.schres.2012.01.038 [DOI] [PubMed] [Google Scholar]
- Pan L. A., Batezati-Alves S. C., Almeida J. R. C., Segreti A., Akkal D., Hassel S., et al. (2011). Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J. Am. Acad. Child Adolesc. Psychiatry 50 602–611. 10.1016/j.jaac.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L. A., Hassel S., Segreti A. M., Nau S. A., Brent D. A., Phillips M. L. (2013a). Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med. 43 2129–2142. 10.1017/S0033291712002966 [DOI] [PubMed] [Google Scholar]
- Pan L., Segreti A., Almeida J., Jollant F., Lawrence N., Brent D., et al. (2013b). Preserved hippocampal function during learning in the context of risk in adolescent suicide attempt. Psychiatry Res. 211 112–118. 10.1016/j.pscychresns.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M., Ehrlich S., De Pisa E., Mann J. J., Innamorati M., Cittadini A., et al. (2007). White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 257 494–499. 10.1007/s00406-007-0755-x [DOI] [PubMed] [Google Scholar]
- Pompili M., Innamorati M., Mann J. J., Oquendo M. A., Lester D., Del Casale A., et al. (2008). Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog. Prog Neuropsychopharmacol. Biol. Psychiatry 32 1501–1507. 10.1016/j.pnpbp.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Radua J., Phillips M. L., Russell T., Lawrence N., Marshall N., Kalidindi S., et al. (2010). Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage 49 939–946. 10.1016/j.neuroimage.2009.08.030 [DOI] [PubMed] [Google Scholar]
- Richard-Devantoy S., Berlim M. T., Jollant F. (2014). A meta-analysis of neuropsychological markers of vulnerability to suicidal behavior in mood disorders. Psychol. Med. 44 1663–1673. 10.1017/S0033291713002304 [DOI] [PubMed] [Google Scholar]
- Rusch N., Spoletini I., Wilke M., Martinotti G., Bria P., Trequattrini A., et al. (2008). Inferior frontal white matter volume and suicidality in schizophrenia. Psychiatry Res. 164 206–214. 10.1016/j.pscychresns.2007.12.011 [DOI] [PubMed] [Google Scholar]
- Ryding E., Ahnlide J. A., Lindstrom M., Rosen I., Traskman-Bendz L. (2006). Regional brain serotonin and dopamine transporter binding capacity in suicide attempters relate to impulsiveness and mental energy. Psychiatry Res. 148 195–203. 10.1016/j.pscychresns.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Scheuerecker J., Meisenzahl E. M., Koutsouleris N., Roesner M., Schopf V., Linn J., et al. (2010). Orbitofrontal volume reductions during emotion recognition in patients with major depression. J. Psychiatry Neurosci. 35 311–320. 10.1503/Jpn.090076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Takahashi Y., Liu T. L., McDannald M. A. (2011). Does the orbitofrontal cortex signal value? Ann. N.Y. Acad. Sci. 1239 87–99. 10.1111/j.1749-6632.2011.06210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff P. H., Pruitt P., Sharma M., Radwan J., White R., Diwadkar V. A. (2012). Structural brain abnormalities and suicidal behavior in borderline personality disorder. J. Psychiatr. Res. 46 516–525. 10.1016/j.jpsychires.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoletini I., Piras F., Fagioli S., Rubino I. A., Martinotti G., Siracusano A., et al. (2011). Suicidal attempts and increased right amygdala volume in schizophrenia. Schizophr. Res. 125 30–40. 10.1016/j.schres.2010.08.023 [DOI] [PubMed] [Google Scholar]
- Strawn J. R., Wehry A. M., Chu W. J., Adler C. M., Eliassen J. C., Cerullo M. A., et al. (2013). Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress. Anxiety 30 842–848. 10.1002/Da.22089 [DOI] [PubMed] [Google Scholar]
- Szamosi A., Nagy H., Keri S. (2013). Delay discounting of reward and caudate nucleus volume in individuals with alpha-synuclein gene duplication before and after the development of Parkinson’s disease. Neurodegener. Dis. 11 72–78. 10.1159/000341997 [DOI] [PubMed] [Google Scholar]
- Szatkowska I., Szymanska O., Marchewka A., Soluch P., Rymarczyk K. (2011). Dissociable contributions of the left and right posterior medial orbitofrontal cortex in motivational control of goal-directed behavior. Neurobiol. Learn. Mem. 96 385–391. 10.1016/j.nlm.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Tanaka S. C., Doya K., Okada G., Ueda K., Okamoto Y., Yamawaki S. (2004). Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat. Neurosci. 7 887–893. 10.1038/nn1279 [DOI] [PubMed] [Google Scholar]
- Tanaka S. C., Schweighofer N., Asahi S., Shishida K., Okamoto Y., Yamawaki S., et al. (2007). Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS ONE 2:e1333 10.1371/Journal.pone.0001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P. E., Eden G. F., Jones K. M., Zeffiro T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16 765–780. 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Turkeltaub P. E., Eickhoff S. B., Laird A. R., Fox M., Wiener M., Fox P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33 1–13. 10.1002/Hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heeringen C. (2012). “Stress–diathesis model of suicidal behavior,” in The Neurobiological Basis of Suicide, ed. Dwivedi Y. (Boca Raton, FL: CRC Press; ), 113–125. 10.1201/b12215-7 [DOI] [Google Scholar]
- van Heeringen C., Bijttebier S., Godfrin K. (2011). Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci. Biobehav. R 35 688–698. 10.1016/j.neubiorev.2010.08.007 [DOI] [PubMed] [Google Scholar]
- van Heeringen K., Mann J. J. (2014). The neurobiology of suicide. Lancet Psychiatry 1 63–72. 10.1016/S2215-0366(14)70220-2 [DOI] [PubMed] [Google Scholar]
- Vang F. J., Ryding E., Traskman-Bendz L., van Westen D., Lindstrom M. B. (2010). Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res. Neuroimaging 183 177–179. 10.1016/j.pscychresns.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Wagner G., Koch K., Schactitzabel C., Reichenbach J. R., Sauer H., Schlosser R. G. M. (2008). Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J. Psychiatr. Neurosci. 33 199–208 [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Koch K., Schachtzabel C., Schultz C. C., Sauer H., Schlosser R. G. (2011). Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage 54 1607–1614. 10.1016/j.neuroimage.2010.08.082 [DOI] [PubMed] [Google Scholar]
- Wagner G., Schultz C. C., Koch K., Schachtzabel C., Sauer H., Schlosser R. G. (2012). Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J. Psychiatry Res. 46 1449–1455. 10.1016/j.jpsychires.2012.07.013 [DOI] [PubMed] [Google Scholar]
- World Health Organisation [WHO]. (2002). World Report on Violence and Health 1st Edn. Geneva: World Health Organisation [Google Scholar]
- Zhang H., Chen Z., Jia Z., Gong Q. (2014). Dysfunction of neural circuitry in depressive patients with suicidal behaviors: a review of structrual and functional neuroimaging studies. Progr. Neuropsychopharmacol. Biol. Psychiatry 4 61–66. 10.1016/j.pnpbp.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Zuckerman M. (1999). Vulnerability to psychopathology: A Biosocial Model. Washington, DC: American Psychological Association [Google Scholar]