Figure 2.
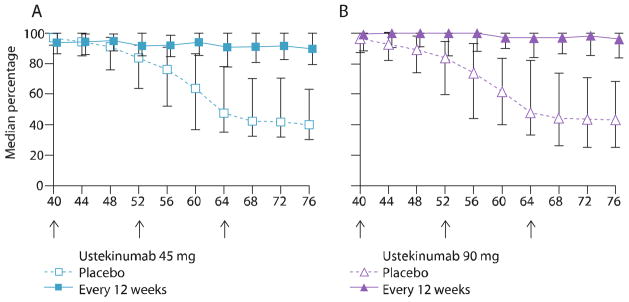
Median percentage improvement in Psoriasis Area and Severity Index (PASI) score from baseline at weeks 40 to 76 for ustekinumab (45 and 90 mg)
(Adapted with permission from Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial [PHOENIX 1]. Lancet. 2008;371:1665–74.)
