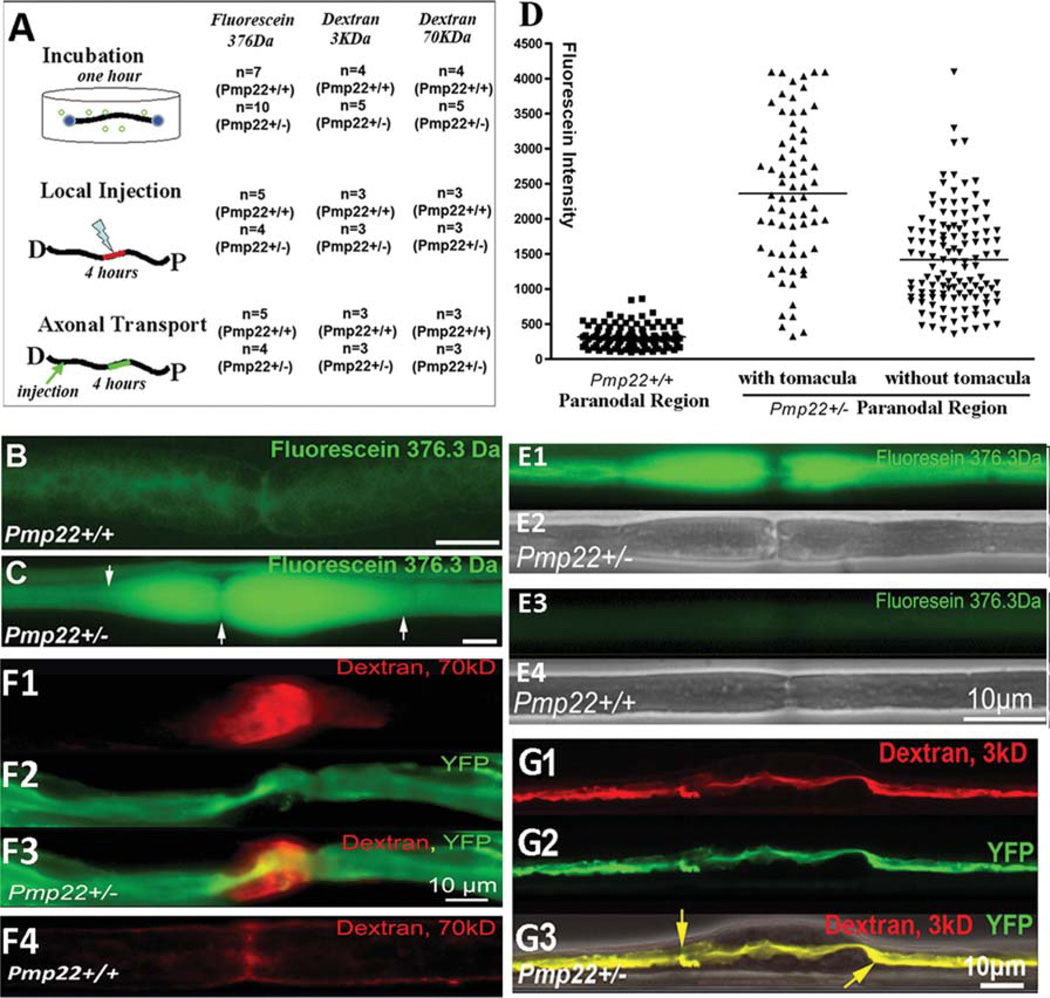
FIGURE 1.
Increase of myelin leakage in Pmp22+/− nerve fibers. Experiments were done in 24 Pmp22+/− mice and 15 Pmp22+/+ mice at 10 to 11 months of age. In addition, the key findings in B–D were replicated in 6-week-old mice (3 Pmp22+/+ and 3 Pmp22+/− mice; E1–4). (A) A diagram shows 3 different procedures that were used to evaluate myelin leakage. In the first procedure, 1cm sciatic nerve fascicles were submerged in artificial cerebrospinal fluid (aCSF; 10mM HEPES, 110mM NaCl, 17.8mM NaHCO3, 4mM MgSO4, 3.9mM KCl, 3mM KH2PO4, 1.2mM CaCl2, and 10mM dextrose) after epineurium removal. Nerves were sealed at both ends with Vaseline. Three different sizes of fluorescence molecules (fluorescein 376Da from Sigma, St Louis, MO; 3kDa and 70kDa dextran from Molecular Probes, Eugene, OR) were added (10mg fluorescein/ml aCSF; 2 or 5 mg/ml for 3 or 70 KDa dextran) for a 1-hour incubation at room temperature without oxygenation. Following a brief wash, the nerve fascicle was fixed in 4% paraformaldehyde for 5 minutes and teased into individual nerve fibers for fluorescence microscopy. In the second procedure, a 10ll Hamilton syringe connected with a glass pipette tip (diameter = 50lm) was used to inject 5ll fluorescence dyes into the sciatic nerves 5mm proximal to the sciatic nerve bifurcation. The injected mice were kept alive for 4 hours while anesthetized with tribromoethanol (Avertin). A 5mm sciatic nerve fascicle with injected dyes was then fixed and teased. In the third procedure, the sciatic nerve was injected with the dyes. The nerve was crushed with #5 Diamond forceps for 5 seconds to allow dyes to enter the transected axons. Mice were kept alive for 4 hours. A 5mm nerve fascicle 10mm proximal to the crushed site was processed for microscopic evaluation. (B) A teased nerve fiber from a Pmp22+/+ mouse showed weak fluorescence after incubation with fluorescein. (C) Fluorescein molecules were drastically increased in Pmp22+/− nerve fibers, particularly in the tomaculous region (between arrows). (D) Fluorescence (fluorescein) intensity in the incubated nerves was quantified as described in Materials and Methods. The intensity was significantly increased in Pmp22+/− nerve fibers compared to Pmp22+/+ nerve fibers (n = 105 Pmp22+/+ nerve fibers from 7 mice, n = 69 Pmp22+/− fibers from 10 mice with counts in tomaculous region, n = 122 Pmp22+/− fibers from 10 mice with counts in nontomaculous region; p< 0.001). (E1–E4) Fluorescein was incubated with nerves from 6-week-old mice. A similar difference of fluorescent intensity was observed between Pmp22+/+ and Pmp22+/− nerves. E2 and E4 were phase-contrast images. (F1–F4) To determine whether Pmp22+/− myelin is permeable to larger sizes of molecules, nerves from mice at 10 to 11 months of age were incubated with 70kD dextran. (F1) Strong fluorescence was visible in the paranodal myelin. (F2) Axon was labeled by yellow fluorescent protein (YFP) that was expressed from a transgene under a neuronal specific promoter.8 (F4) In contrast, fluorescence in Pmp22+/+ nerve fibers was minimal. (G1–G3) The 3kD dextran was taken up by the transected axons and transported in retrograde. The dextran was confined within the Pmp22+/− axon 10mm proximal to the transected site, including the axon within the tomaculous region (between arrows in G3). (G3) Axon was labeled by YFP overlapped with dextran, suggesting no fluorescence molecules leaking out of the axon. An overlaid phase-contrast image shows the location of tomacula.